Achiko’s President, Dr Morris S. Berrie, explains how the company’s innovative AptameX™ technology could offer a sustainable solution to mass COVID-19 testing.
Since the outbreak of COVID-19, the efforts of the global scientific community have been nothing short of outstanding. The world has witnessed unprecedented development in vaccine technology. AstraZeneca / Oxford produced the world’s first vaccine in a not-for-profit endeavour, whilst Pfizer-BioNTech and Moderna, both followed with first-in-class mRNA technologies. All three were approved and in circulation within a year. Vaccination programmes offer the promise of curbing COVID-19, yet governments and businesses are increasingly accepting what epidemiologists have long warned: the pathogen will circulate for years, or even decades, leaving society to coexist with the virus, much as it does with other endemic diseases such as flu and measles.
Achiko AG believe that testing has become essential. The need to be proactive in detecting a positive result is increasingly critical, especially in the developing world. Whilst the RT-PCR test is rightly considered the gold standard for accurate testing, the explosion of lateral flow/ rapid testing has been in the news recently for the wrong reasons creating additional worries surrounding the reliability and accuracy of such test results. False positives and false negatives create their own challenges but the issue surrounding the need for accurate testing also now centres around what Ct score the sensitivity results are reported. The rapid test kits most widely used in UK universities, schools, and care homes have been reported to detect just 48.89% of COVID-19 infections in asymptomatic people when compared with PCR test.
The ease with which the coronavirus spreads, the emergence of new virulent strains, most recently the Indian (Delta) variant, and poor access to vaccines in large parts of the world has meant COVID-19 could shift from being recognised as a pandemic disease to an endemic one. The long-lasting and far-reaching effects clearly indicate lasting adaptations to both personal and societal behaviour. Epidemiologists and economists agree; the global socio-economic impact of COVID-19 is undeniable.
Endemic COVID-19 does not necessarily mean continuing coronavirus restrictions, largely because vaccines are so effective at preventing severe disease and slashing hospitalisations and mortality rates. Hospitalisations have fallen to negligible numbers in the UK following the double vaccination programme, with deaths having plummeted to zero and now, even with the emergence of the new Delta variant in April, daily deaths remain below double-digit growth months later. And yet, the insidious nature of the virus remains prevalent – testing regularly would offer a way to control the spread of the virus and its variants whilst the vaccination programme continues apace.
Organisations need to plan for a long-term future in which prevention methods such as masking, good ventilation and testing continue. It is the testing space that Achiko have applied disruptive thinking to the problem. Long before the debate centred on ‘travel passports’ Achiko undertook to adapt and redeploy its financial wallet technology known as Teman Sehat™ to develop a digital passport specifically as an application for COVID-19 testing. Subsequently we have harnessed the diagnostic capability of DNA-aptamer technology (nucleic acid aptamers for target validation and therapeutic applications).
AptameX™ is a fast, accurate, low-cost diagnostic test for the SARS-CoV-2 (COVID-19) virus protein in saliva samples using gold nanoparticle-DNA aptamer technology and a UV-VIS quantitative spectrophotometer detection system. When connected to the Teman Sehat mobile phone result service, testing is conducted within 15 minutes.
Aptamers are a special class of nucleic acid molecules that are increasingly being investigated for clinical use. These small RNA/DNA molecules can form secondary and tertiary structures capable of specifically binding proteins or other cellular targets; they are essentially a chemical equivalent of antibodies.¹
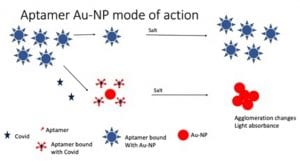
Aptamers are short, single-stranded oligonucleotides capable of binding various molecules with high affinity and specificity. Currently, monoclonal antibodies (mAbs) are routinely used for the diagnostics of various diseases. Achiko’s science is based upon the use of a DNA-aptamer sequence that has a specific strong affinity for the COVID-19 S1 protein spike. When the DNA-aptamer is bound to gold nanoparticles the resultant conjugate cleaves in the presence of the COVID-19 viral protein. The subsequent increased free gold concentration is detectable using a cuvette colorimetric assay performed in a UV-VIS spectrophotometer and the diagnostic test result is automatically sent to the mobile phone.
Aptamers are known to recognise many viruses, including the human immunodeficiency virus (HIV), hepatitis C virus (HCV) and influenza virus.² The aptamer generation protocol SELEX was developed over 20 years ago, yet therapeutic aptamers are rare, e.g. Macugen (Pegaptanib) binds to the vascular endothelial growth factor (VEGF) and blocks abnormal angiogenesis in the eye, preventing intraocular hemorrhage and loss of vision was approved for therapeutic application over a decade ago.³
Achiko recognises that aptamers offer an exciting prospect in the world of diagnostics as they can be raised against any kind of target, including toxic or non-immunogenic ones. Aptamers are synthetic molecules that combine the advantages typical both of low-molecular-weight substances and proteins and can be used under non-physiological conditions for analytical and diagnostic purposes. They bind their target with high affinity similar to, or higher than, antibodies. Additionally, they are 10-fold smaller than antibodies and can be chemically modified at will in a defined and precise way. Importantly they can be easily delivered and demonstrate high tissue penetration similar to that of small molecules. They can be stored with a shelf-life of six to 12 months, and both sourcing and production is guaranteed with high batch-to-batch reproducibility. Compared to antibody / antigen production, synthesis is cheap and hugely scalable.
Achiko is addressing the need to control the spread of the pandemic head on through its proprietary AptameX test kit and genuinely instilling confidence. We recognise that vaccines are making incredible inroads to containing the disease, but it is likely to take a years for the current situation to stabilise. Mass testing must be at an optimum price / convenience / performance point for all populations. These critical criteria are still not met by RT-PCR or lateral flow rapid tests (antibody production is a lengthy and expensive process; and there is a ceiling to production capacity).
Achiko has a real opportunity to affect global change and particularly at a low-price point by providing a COVID-19 test that is:
- Typically, more accurate than lateral flow tests (and much easier to use, which will improve both take-up and compliance);
- Typically, lower cost than PCR tests; and
- Practically limitless in capacity, as it is based on synthetic DNA.
Steven Goh, Chief Executive Officer of Achiko said: “We feel it is important that society moves away from reactive testing, not least because the actual infectious stage of viral infections is often asymptomatic. At Achiko, we advocate moving towards proactive testing integrated into your daily routine and one that is wholly unobtrusive. It is our belief that alongside vaccination, regular testing will be required to see employees return to work with alacrity. Equally, travel will only be permitted with requisite proof of a negative COVID-19 test. The AptameX test is non-invasive and ergo that means that this additional layer of protection against the spread of the disease does not have to be intrusive.”
The problems however are apparent. Recently the US Food and Drug Agency (FDA) raised concerns about the safety and the marketing of rapid lateral flow COVID-19 tests, which incidentally are the cornerstone of the UK’s mass testing programme. On 10 June 2021, the FDA warned the public to stop using the Innova SARS-CoV-2 antigen rapid qualitative test for detecting infection and suggested the tests should be destroyed and binned or returned to the manufacturer. The FDA published a class 1 recall (defined as serious injury or death) after an investigation uncovered “significant concerns that the performance of the test has not been adequately established, presenting a risk to health.” Cheap testing is one thing, but cheap accurate and reproducible testing another entirely.
In addition, there is a worry that the Delta variant whilst not only being more transmissible is more infectious than the Alpha variant. It is being suggested that the infective value, R0 may be around 5, and possibly as high as 7. Around 25 variants are currently under monitoring and eight specifically under investigation in the UK.
Achiko believes that there will be greater emphasis put on personal risk and responsibility, and in places such as malls, restaurants etc. people will be required to use personal judgment to wear masks. In tightly confined spaces, people will likely still be required to wear face coverings but additionally to demonstrate a negative test result.
“Achiko feels strongly that the need to bring back confidence, to open businesses and allow people to go about their day-to-day lives is of critical importance; to do that, we need accurate testing that meets not only WHO standards but importantly those of the likes of the FDA and EMA. To that end, the Achiko team has been working around the clock to produce a reliable diagnostic test that helps contain the spread of the novel COVID-19, in order that we help improve the livelihood and socio-economic impact of populations severely impacted by this deadly disease,” said Dr Morris S. Berrie, President of Achiko. “We are excited at the impact we can have, not least in those parts of the world that are most in need.”
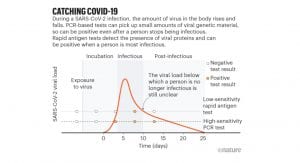
Sensitivity and CT value
Currently, the performance of rapid antigen tests is validated against RT-PCR, the gold standard in COVID-19 testing because it can amplify signals from small quantities of viral particles. The pre-amplification cycles quantified as a cycle threshold value (Ct value) is a semi-quantitative indicator of viral load or the amount and concentration of viral genetic material in the patient sample. But the challenge is that within different PCR systems, Ct values are not directly comparable across laboratories.
The performance of antigen-based rapid diagnostic tests varies greatly with clinical sensitivities ranging from 23% to 71% and 2-5 orders of magnitude less sensitive than RT-PCR.⁴ Current rapid tests have high sensitivities at Ct values of 25 or lower, but at higher Ct values particularly above 28, performance declines.⁵
Optimal Ct values should be lower than 25. A case in point: Innova tests “identified only two-thirds of the cases with Ct levels below 25” indicates that the laboratory which processed the samples performed at a level far from what should be required.⁵ Their Ct value of 25 would probably be equivalent to a Ct of 30 or higher if another lab would have processed the same samples. The key message is that a third of the cases that were missed were probably infectious. If rapid tests should perform consistently at Ct values lower than 25, given the differences across laboratories, what should the Ct cut-off be so that diagnostic performance can be guaranteed?
Mak et al, compared three different antigen tests and defined a “high” viral load at mean Ct value of 15.27-16.70 across the three commercially available rapid tests; “normal” at 23.21-24.77 and “low” at 31.83. ⁴ Rapid tests can then be characterised across the three ranges of High, Normal and Low to understand its performance through its sensitivity curve. Thus, even if Ct values do not match across different laboratories, the trend will be apparent, and the test’s performance can be understood at either side of a given Ct value.
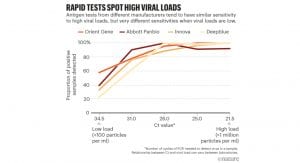
In assessing the performance of AptameX in its validation trials, Achiko considered Mak et al and report that the Pekan Baru trial undertaken at a mean Ct value of 28.3 exhibited a sensitivity of 77.6%. We expect that validation trials conducted where the mean Ct value would be 25 or lower, will demonstrate the sensitivity of AptameX as being significantly higher.
The issue of Ct scores is now paramount. Rapid antigen test already in the market report published low sensitivities (circa 11%) at a Ct value of around 32. At a Ct of 32, i.e., very low viral load, Achiko reports the sensitivity of AptameX as 75.69%.⁴
Achiko is assessing patients with higher viral loads to be able to report performance at Ct values less than 25. We strongly believe that when AptameX is tested on the same basis as other rapid tests and against a population of COVID-19 positive cases identified with a Ct value in the same range as other tests, AptameX is likely to pass the WHO guidelines of 70% for sensitivity and 95% of specificity.
The rationale for the use of rapid antigen tests is to be able to detect the SARS-CoV-2 virus in patients with high viral loads and isolate them when they are contagious and thereby curb the pandemic. From a public health standpoint, in addition to accuracy, the other factor to consider with rapid tests is their frequent use. Even if all the cases can be correctly identified, other questions around infectiousness remain: degree and stage of disease, whether they have symptoms, and the infection rates in the geographical area of the patient concerned. Any test should ideally capture those that are at either side of the curve, i.e., those with early symptom on-set who will be infectious and those in recovery who are still shedding the virus. But as yet, the duration of infectiousness is not fully understood. A single test gives an answer at a single point in time and cannot be the basis of re-opening businesses and in controlling the pandemic. Testing should be frequent, done at least twice a week, to make these rapid tests effective in a pandemic.
Conclusion
At a low-price point, and already exhibiting comparable sensitivity to existing rapid tests, AptameX aims to be the solution in frequent global mass-testing.
Pending further clinical validation to assess the AptameX COVID-19 test kit at relevant published Ct values of less than 25, we report that AptameX performs to a comparable level when compared to other rapid antigen tests available in the market. Achiko has achieved 77.6% sensitivity at a favourable Ct score of 28.3 conducted in Indonesian field testing. Achiko is now in the process of undertaking selectivity and stability studies in Europe as a precursor to seeking full CE mark registration in late Q3/early Q4 2021.
Achiko is excited for the future; we are moving to complete full product registration imminently in Indonesia. Achiko recently shipped its first AptameX product and production has commenced on follow-up test kits in Singapore (initial capacity to diagnose 10,000 patients per day) with the aim to expand production capacity in Indonesia itself.
Achiko and PT Indonesia Farma Medis (IFM) have established a joint venture company PT Achiko Medica Indonesia for the production, distribution, and marketing of AptameX. The initial roll out of AptameX and mobile app Teman Sehat in Indonesia will commence in early Q3 2021, providing diagnostic assurance services against the pandemic.
“We believe that the current COVID-19 environment presents a concern for everyone, and providing a low-cost, comparably accurate testing service, is a solution to restore confidence in workplaces, amongst consumers, tourists, and within manufacturing,” said Steven Goh, CEO of Achiko. “We believe that working together with our partners, reaching just 2% of Indonesia’s population of 270 million people spread over 17,000 islands may be possible later this year.”
These milestones for Achiko underscores the company commitment to developing a rapid, accurate, cost-effective solution to diagnose those with COVID-19. The investments Achiko have made in transitioning from a fintech to a medtech diagnostic company are beginning to show promise; the technologies of AptameX and Teman Sehat™ offer an extraordinary solution to managing further outbreaks of COVID-19. Achiko can respond and act swiftly to the global public health crisis, not least in developing countries.
References
1 X. Ni, M. Castanares, A. Mukherjee, S.E. Lupold, Curr Med Chem. 2011; 18(27): 4206–4214
2 Aptamers in virology: recent advances and challenges. Binning JM, Leung DW, Amarasinghe GK Front Microbiol. 2012; 3():29
3 Pegaptanib: in exudative age-related macular degeneration. Siddiqui MA, Keating GM Drugs. 2005; 65(11):1571-7; discussion 1578-9
4 ‘Analytical sensitivity and clinical sensitivity of the three rapid antigen detection kits for detection of SARS-CoV-2 virus’, Gannon C.K. Mak et al, Journal of Clinical Virology, 29 Oct 2020 www.sciencedirect.com/science/article/pii/S1386653220304261
5 ‘Rapid coronavirus tests: a guide for the perplexed,’ G. Guglielmi, Nature, 11 February 2021. www.nature.com/articles/d41586-021-00332-4
6 ‘Evaluation of rapid antigen detection kit from the WHO Emergency Use List for detecting SARS-CoV-2”, Gannon C.K. Mak and others, Journal of Clinical Virology, 4 December 2020. www.sciencedirect.com/science/article/pii/S1386653220304546
7 ‘Rapid coronavirus tests: a guide for the perplexed,’ G. Guglielmi, Nature, 11 February 2021. www.nature.com/articles/d41586-021-00332-4 adapted from A. Crozier et al. Br. Med. J. 372, n208 (2021)
This article is from issue 18 of Health Europa. Click here to get your free subscription today.

























