The high and increasing unmet patient need in diabetic kidney disease demands new drugs. Betagenon AB has the answer.
Globally, approximately 40% of Type 2 diabetes (T2D) patients develop diabetic kidney disease (DKD), and diabetes is the leading cause of end-stage renal disease (ESRD), requiring dialysis or a kidney transplant. The increasing prevalence of DKD parallels the dramatic worldwide rise in prevalence of diabetes, which is caused by the global pandemic of obesity.
DKD is a microvascular disease, and the natural history of diabetic kidney disease includes glomerular hyperfiltration, progressive albuminuria, declining glomerular filtration rate (GFR) and ESRD. DKD is also strongly linked to hyperglycaemia, hyperinsulinaemia and insulin resistance, which are in turn associated with glomerular hypertrophy, inflammation and kidney fibrosis.
Standard of care for DKD is:
- Control of blood sugar levels as part of regular diabetes care; and
- Antihypertensive medication with ACE inhibitors (ACEi) and angiotensin II receptor blockers (ARB) drugs that target the angiotensin II system and cause haemodynamic changes and reduce hypertension in the kidney.
Unfortunately, however, the current treatment of DKD shows limited efficacy, and despite decades of research on the various mechanisms such as fibrosis and inflammation, no new drugs have been approved in the last 15 years for the treatment of DKD or chronic kidney disease (CKD). Thus, there is a great need for novel, more efficient drugs to prevent and treat DKD and CKD.1
Glomerular hyperfiltration
Glomerular filtration is an essential process whereby fluid in the blood is filtered by the kidneys. Glomerular ‘hyperfiltration’ mediated by intraglomerular hypertension is common in the early stages of T2D and believed to provoke a later decline in globular filtration rate (GFR), ultimately potentially leading to ESRD. The balance shifts to glomerular hyperfiltration as a result of high intraglomerular pressure caused by increased blood flow into and decreased blood flow out from the glomerulus. ACEi/ARB drugs affect glomerular hypertension and hyperfiltration by increasing the blood flow out from the glomerulus.
SGLT2 inhibitors are recently introduced anti-hyperglycaemic agents that show improved renal outcome in T2D patients with established cardiovascular disease by reducing estimated globular filtration/hyperfiltration (eGFR) via activation of tubuloglomerular feedback that reduces blood flow into the glomerulus. However, SGLT2 inhibitors are contra-indicated in T2D patients with impaired renal function due to lack of anti-glycaemic effect in this group of patients.2
AMPK as a target for T2D and cardiovascular complications
AMP-activated protein kinase (AMPK) controls energy status at both the cellular and whole organism levels. As a central regulator of energy homeostasis, it co-ordinates metabolic pathways and thus balances nutrient supply with energy demand. It is activated when energy levels fall, e.g. by exercise and caloric restriction, and hence delivers beneficial metabolic and cardiovascular effects. To restore energy charge, activation of AMPK in the brain increases food intake. In skeletal muscle and other tissues/organs, AMPK stimulates energy-generating processes such as glucose uptake and fatty acid oxidation. Increased AMPK activity also increases blood flow to provide target tissues with oxygen and nutrients like glucose and lipids. Thus, AMPK is considered to be an important therapeutic target for prevention or cure of T2D and associated cardiovascular disease (CVD).3
AMPK and DKD
AMPK is also a major regulator of glucose and lipid metabolism and of tubular transport of water and ions in the kidney. Multiple interventional studies in animal models have suggested potentially beneficial effects of AMPK activation in diabetic kidney disease.4 Notably, caloric restriction in T2D patients with abdominal obesity, known to activate AMPK, ameliorates glomerular hyperfiltration, blood pressure, blood glucose levels, insulin sensitivity, and other cardiovascular risk factors, like serum levels of angiotensin II and albuminuria – effects that might translate into cardiorenal protection.5
AMPK activator O304 as a novel potential treatment of T2D, CVD and DKD.
Preclinical development
Betagenon’s O304 is a direct PAN-AMPK activator that does not enter the brain. In preclinical models of hyperglycaemia/diabetes, O304 increases glucose uptake in skeletal muscle, reduces insulin resistance and promotes β-cell rest. O304 increases energy expenditure and prevents/reduces obesity. Like exercise, O304 lowers blood pressure and increases microvascular perfusion, activates AMPK in the heart, increases cardiac glucose uptake, reduces cardiac glycogen levels, and improves left ventricular stroke volume and endurance. Further, O304 does not cause cardiac hypertrophy in mouse or in rat.6
Thus, O304 exhibits a combination of beneficial metabolic and cardiovascular effects not observed with any other available anti-diabetic drug.
Clinical development – T2D and CVD
O304 is currently the only direct AMPK activator in clinical development. In an exploratory 28-day phase IIa clinical trial in T2D patients, O304 reduced fasting plasma glucose levels and insulin resistance, lowered systemic blood pressure, and increased microvascular perfusion.6 These effects are consistent with results in preclinical studies.
Diabetic kidney disease
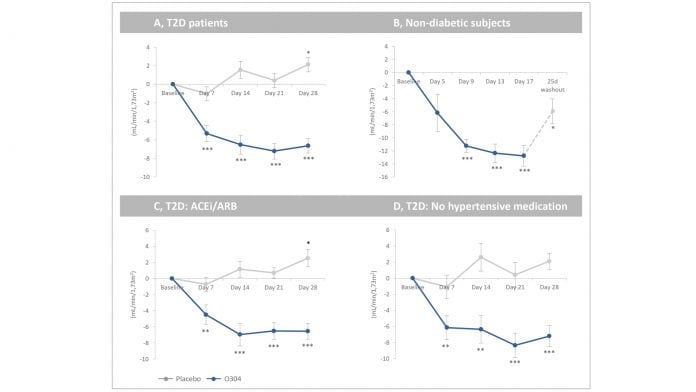
Glomerular hyperfiltration is mediated by changes in microvascular blood flow that cause renal hypertension. In T2D patients, O304 both lowered systemic blood pressure and increased microvascular perfusion. Moreover, post hoc analyses of phase I and IIa clinical trials of O304 show that O304 potently reduced eGFR by a rapid, stable and reversible haemodynamic effect both in T2D patients on metformin and in obese non-diabetic subjects.
O304 also reduced eGFR both in T2D patients with no anti-hypertensive treatment or on top of ACEi/ARB drug treatment (standard of care for DKD), indicating that O304 either reduces eGFR by the same mechanism but more efficiently or by a different mechanism than ACEi/ARB drugs. Thus, O304 reduces blood glucose and insulin resistance, lowers blood pressure, increases microvascular perfusion, and reduces renal filtration/hyperfiltration in T2D patients – beneficial metabolic and haemodynamic effects that are likely to translate into long-term protection of kidney function and thus reduced incidence of diabetic kidney disease.
O304 and SGLT2 inhibitors in combination as treatment for T2D and DKD
SGLT2 inhibitors fail to show anti-glycaemic efficacy in T2D patients with impaired renal function and are therefore contra-indicated in this group of patients. However, O304 and SGLT2 inhibitors in combination potently and synergistically reduce hyperglycaemia, hyperinsulinaemia and insulin resistance in diet-induced obese mice, indicating that the combination of these two classes of compounds may both improve glucose homeostasis and prevent DKD in T2D patients in a potent manner. The recently identified subgroup 3 of T2D patients who are obese (BMI ~35), insulin resistant and hyperinsulinaemic, and have a five-fold higher risk of developing DKD and currently lack efficient treatment, may in particular benefit from such a combination therapy.7
Clinical development of O304 for T2D, DKD and CKD in progress
The effects of O304 alone, as well as in combination with a SGLT2 inhibitor, on glucose homeostasis, blood pressure, microvascular perfusion and renal hyperfiltration will be monitored in a six-month phase IIa clinical trial in obese and insulin-resistant T2D patients, as well as in non-diabetic insulin-resistant subjects with central obesity, at risk of developing CKD.7
About Betagenon AB
Betagenon AB is a privately owned Swedish biotechnology company.
Betagenon AB has received funding from the EU’s research and innovation framework programme Horizon 2020 (EU project 754268 – AMPK-DIAB).
Contact thomas.edlund@betagenon.com.
References
- Tuttle, Diabetes 66:14-16, 2017
- Heerspink et al., Circulation 134:752–772, 2016
- Day et al., Trends in Endocrinology & Metabolism 545-560, 2017
- Salatto et al., J Pharmacol Exp Ther 361:303–311, 2017
- Ruggenenti et al., Diabetes 66:75–86, 2017
- Steneberg et al., JCI Insight, in press 2018
- Ahlqvist et al., Lancet Diabetes Endocrinol. 6:361-369, 2018
Thomas Edlund
Betagenon AB
+46 90154822
info@betagenon.com
www.betagenon.com
This is a commercial article that will appear in Health Europa Quarterly issue 6, which will be published in August, 2018.

























