
Its unparalleled cannabis-based products and ongoing commitment to rigorous scientific research make Columbia Care LLC a world leader in medical cannabis.
CEO Nicholas Vita talks us through the world of medical cannabis and how Columbia Care LLC is striving to be the world leader in such a controversial yet rapidly growing industry.
The largest challenges for the marketing of effective medical cannabis-based products in any nation or on a global scale are the standardisation of the product that patients are taking, providing peer-reviewed data to inform utilisation, and the availability of population-level economic impact analysis to facilitate payer adoption. Unfortunately, the most fundamental factors that would unlock all three of these foundational pillars, the methods and procedures to assure that patients receive the same dose of the same chemicals each time and that they result in a consistent biological effect, have not been a focus of the cannabis industry to date.
The fact that there are thousands of combinations of cannabinoid molecules that are present in final cannabis products and conventional wisdom accepts ‘strains’ (i.e. raw or extracted agricultural material using ‘street’ names) as an acceptable medical option has led to an underestimation of the variability, inconsistency and often unpredictability in the available products.
Columbia Care
Notwithstanding, one medical cannabis provider, Columbia Care, one of the market leaders in the United States, has built its company with the tenet that patients have the right to receive natural, plant-based, standardised products that retain the benefits from combinations of multiple, targeted cannabinoids in a precise, unit-dosed pharmaceutical formulation and format to regulate each treatment. Columbia Care’s efforts are opening the door for bona fide cannabis research that can withstand the scrutiny of peer review, enabling comparative analysis to the standard of care, and validation of efficacy, assessment of risks (such as polypharmacy) and population-based economic analysis to quantify the benefits of these medicines for providers, policymakers and patients.
Founded in 2013, Columbia Care is one of the largest and most experienced providers of medical cannabis-based products and services in the United States. According to management’s estimates, Columbia Care’s US licences permit it to reach over 50% of the US adult population (Fig. 1). Further, upon completion of final regulatory steps, Columbia Care will be authorised to cultivate, process and distribute medical cannabis in Malta and export medical cannabis throughout Europe.
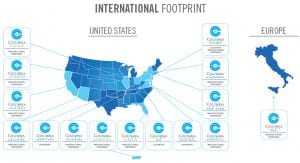
The company’s focus on innovating and validating novel medical cannabis-based products and patient care has resulted in a continually expanding portfolio of patented formats and formulations that, today, encompasses over 20 Columbia Care brands. The company has the capability to serve its patients in a direct and relevant way by leveraging its longitudinal patient database.
Columbia Care’s R&D teams continually ‘translate’ the data they receive from consenting patients every day and overlay it with real-world data (RWD), patient-reported outcome measures (PROMs) and sponsored study data, all of which have been gathered since the company’s founding over six years ago. As a result, Columbia Care can utilise this evidence-based patient feedback loop to ensure its product formulations are optimised for the intended benefits and effects desired to address the specific categories of conditions and symptoms that matter most to patients.
Columbia Care’s primary goal is to provide the highest quality and most consistent cannabis-based medical and wellness products available globally. To meet its standardisation criterion, Columbia Care has demonstrated the ability to achieve active pharmaceutical ingredient (API) variability of <1%, which is significantly below the US Food and Drug Administration’s 10% benchmark for API variability for pharmaceutical products sold in the US. It does not offer products using floss or flower under the Columbia Care name, as those products can have widely different cannabinoid contents on sequential harvests despite sharing the same genetic make-up (Fig. 2).

Products
The ability to ensure product consistency from lot to lot has enabled Columbia Care to file for patent protection on four principal combination product brands, TheraCeed™, ClaraCeed™, ClaraCeed Ultra™ and EleCeed™, with plans for a number of additional product patent filings to follow (Figs. 3, 4). These brand architectures are combinations of not only THC and CBD but of a minimum of ten precisely engineered cannabinoids, as well as terpenes and flavonoids. These precisely formulated products are engineered to include the cannabinoid types and amounts that data has indicated are most effective for particular conditions and symptoms where cannabinoids have shown efficacy.


The condition-specific products that Columbia Care manufactures and continues to develop have been constructed from the evidence that exists in the public domain (National Academies of Sciences, Engineering, and Medicine, 2017). Additionally, Columbia Care has applied the knowledge it has gleaned from its own database of patient-reported outcome measures, which includes data from consenting patients taking well-characterised combinations of cannabinoid mixtures since 2013.
Management believes this is one of the most robust datasets in the world in this field, with data currently stemming from the over 50,000 compliant patient transactions the company processes per month conducted at Columbia Care’s network of dispensaries throughout the US. This volume of data is constantly accelerating in number in concert with the company’s overall growth.
Members of the scientific advisory board at Columbia Care, chief scientific officer Rosemary Mazanet, MD, PhD, trustee at University of Pennsylvania Pearlman School of Medicine, and Carlos Bustamante, PhD, professor and chair of biomedical data science at Stanford University, have been tracking RWD to assess real-world evidence (RWE) regarding patient use and purchasing of Columbia Care products. Columbia Care’s data analytics program has been solidifying relationships with global technology leaders to further analyse this RWD, which has been used to construct and optimise the combination products sold now as well as inform the development of new products. In addition to the specific cannabinoid combinations, the product formulations and formats the company currently offers are critical to maximising patient benefit:
- Long-acting formulations: Many patients with chronic conditions require and seek relief from symptoms on a continual basis to manage their conditions, rather than simply responding to breakthrough events. Columbia Care developed and commercialised its patent-pending hard-pressed tablets that combine targeted API combinations with drug delivery technologies to offer patients the best solution to this demand. This combination of highly crafted agricultural strains, exacting chemistry and novel drug delivery technologies allows for greater bioavailability than is observed with oils and enables patients to reduce the number of required doses per day while simultaneously providing longer symptom relief and reducing cost
- Mid-acting formulations: To strike a balance between onset of action and duration of action, Columbia Care has developed and commercialised a number of products that include its proprietary combinations of APIs in formats that can be absorbed through the oral sub-mucosa. Tinctures are one such example of products designed to strike this balance of precision, duration and onset. In an effort to ensure that the benefits of Columbia Care products are being maximised and patient needs and preferences are being addressed, provisional patents have been filed for several other formats that strike this balance
- Immediate relief formulations: To ensure patients have the option to manage breakthrough events or address acute symptomatic episodes, Columbia Care has developed and commercialised a portfolio of proprietary, rapid relief methods of administration. These are products that leverage chemistry, engineering and device technology (proprietary hardware and software), where the precisely manufactured combinations of cannabinoids are absorbed across mucous membranes, provide immediate relief and, in some formats, can be adjusted remotely and monitored in real time by providers to accommodate patient needs. Examples of such products using this route of administration are unit dosable inhaled vaporisation solutions that are delivered by Columbia Care’s proprietary devices.
These products are engineered to deliver and monitor doses tailored specifically to a patient’s and/or provider’s requirements. Other patented products designed to maximise onset of action are Columbia Care’s oral dissolvable tablets that are placed under the tongue to provide rapid mucosal delivery in a reliable and discreet manner. For disabled, female or pediatric patients, separate unit dosable rectal and vaginal suppositories have also been developed for immediate and localised release.
Product innovation
In addition to its current portfolio of internally developed, proprietary formulated medicines, Columbia Care is continually innovating new products and delivery formulations based on in-licensing opportunities with global corporate leaders, research institutions and collaborative initiatives with specialty patient advocacy organisations.
One example of such a partnership is Columbia Care’s licensing agreement with Australia-based nasal respiratory company Rhinomed (ASX:RNO) for the collaborative development of nasally delivered cannabis-based medicines. This partnership affords Columbia Care the ability to pursue one of the most significant unmet medical needs in the developed world: sleep disorders. Sleep apnoea and anxiety-related sleep disorders are just two of the company’s focus areas to improve patient treatment and wellness. The partnership with Rhinomed underscores Columbia Care’s commitment to being the global partner of choice for medical device manufacturers and product developers. The result of this collaboration will be the combination of two validated approaches to addressing sleep apnoea into one drug-eluding device, leveraging Columbia Care’s formulation, manufacturing and distribution capabilities with a market-leading medical device that enables local (nasal mucosa) administration to optimise the delivery of consistent doses of Columbia Care’s pharmaceutical-quality cannabis-based medicine.
In the first quarter of 2019, Columbia Care is launching a patent-pending precision-dose vaporiser technology that aerosolises its medical cannabis formulations for inhalation. This inhalation device provides titratable, dose-metered cannabinoids with consistency of greater than 99%. The challenge for physicians, pharmacists and patients with current vape pens is that the cannabinoid dose that patients receive is entirely dependent on and varies with each inhalation. With this device, the cannabinoid dose is controlled, so patients know how much they are taking and receive a consistent dose with every inhalation.
The nexus of this opportunity surfaced through a multiyear development partnership with Arizona State University, which invented a new, more efficient technology that allows every patient to receive the same amount of medication each time the device is used. Columbia Care believes this method of delivery will be key to ensuring optimal dosages and better outcomes for its customers and will represent a paradigm shift in consumer expectation for performance.
Ongoing research
The best cannabinoid product combinations may still be undiscovered, and most existing formulations require additional validation and testing across a range of therapeutic categories and symptoms. Because of this, Columbia Care is dedicated to conducting rigorous evidence-based research that studies and validates the effects of its precisely manufactured cannabis-based medicines for the treatment of varying indications in high-impact conditions.
This is the hallmark of how Columbia Care fulfils its mission of patient care, builds product awareness, enhances adoption and continually reaffirms its innovative leadership. Among the many areas of study the company is engaged in are the highlighted efforts below.
Opioid abuse
Columbia Care is collaborating with Columbia University on research led by Dr Arthur Robin Williams, MD, MBE, assistant professor in the Division on Substance Use Disorders in the Columbia University Department of Psychiatry, to observe opioid use in patients who start using Columbia Care’s formulated pharmaceutical products.
The study is the only US National Institute on Drug Abuse (NIDA, a subordinate organisation of the National Institute of Health, NIH) grant focusing on the impact access to precisely dosed medical cannabis medicines has on opioid addiction. It will expand upon pilot data collected by Columbia Care, which tracked opioid use in neuropathy patients in New York State after they started using Columbia Care’s dose-metered cannabinoid medicines. This pilot study showed that 62% of these patients decreased or stopped their opioid use while taking TheraCeed, ClaraCeed or EleCeed consistently over a nine-month period.
These pilot results were the basis for the NIDA funding for the larger study and represented the first time the US Government agreed to fund a study in which a US corporate entity such as Columbia Care serves as the product manufacturer.
Columbia Care is also collaborating with New York University (NYU) Langone Medical Center to supply treatment alternatives for patients who are addicted to opioids.
Intractable epilepsy
Columbia Care products ClaraCeed and ClaraCeed Ultra are being used in an observational study in collaboration with The Center for Discovery (TCFD), an internationally renowned provider of advanced specialty care to individuals with complex medical and behavioural conditions. The study is designed to enrol 50 TCFD patients with drug-resistant epilepsy in accordance with current New York State Department of Health guidelines and is led by neurologist Orrin Devinsky, MD, director of NYU Langone’s Comprehensive Epilepsy Center and world leader in the study of epilepsy, and Dr Terry Hamlin, associate executive director of TCFD.
This study will evaluate the optimal dose for safety, tolerability and potential reduction of convulsive seizures using ClaraCeed and ClaraCeed Ultra medical cannabis as an adjunct to their ongoing treatment in subjects with drug-resistant epilepsy. This partnership highlights Columbia Care’s dedication to evidence-based research validating the therapeutic benefits of its precisely manufactured cannabis-derived medicines.
Mental health
The effects of Columbia Care’s pharmaceutical-quality products on mental health will be studied in association with King’s College London, UK. Columbia Care products will be used in three upcoming placebo-controlled multinational studies led by King’s College London and supported by the Wellcome Trust:
- In patients at high risk for psychosis
- In patients with unresponsive psychosis in addition to traditional medicines
- In patients with hard-to-treat psychosis in addition to traditional medicines.
Rheumatoid diseases
Columbia Care is collaborating with the WestMed Medical Group on a therapeutic study of the effectiveness and safety of medical cannabis in the outpatient rheumatologic population.
Sickle cell disease
In collaboration with the University of Pittsburgh, Columbia Care products will be used to assess pain control and inflammation reduction in sickle cell disease.
Patient/genetic profiles
In association with Scientific Advisory Board (SAB) member Bustamante, Columbia Care continues to leverage its ongoing research efforts and existing repository of medical cannabis patient and consumer data to drive product innovation and create targeted predictive outcome models. These capabilities inform users and physicians how to enhance the benefits of Columbia Care products and have a profound positive impact on the way healthcare and consumer analytics are leveraged responsibly. This work positions Columbia Care as a global leader in both the healthcare and consumer goods sectors.
Talent
Columbia Care has assembled a world-class executive leadership team with a diverse set of experiences and backgrounds who have powered the company to its status as a leader in the industry. It was co-founded by Nicholas Vita, current chief executive officer of Columbia Care, and Michael Abbott, current executive chairman. Vita and Abbott collectively possess decades of experience in finance leadership roles from which they are able to draw to inform the continued success of this company globally.
Executive leadership team
The Columbia Care leadership team is comprised of seasoned executives from medicine, healthcare, research, data analytics, marketing, finance, law, operations and risk management, who are passionate about the role that medical cannabis can play in solving some of the world’s most challenging unmet medical needs. The specific expertise of each member of the team has been critical to the success of Columbia Care, and the team is unparalleled in the cannabis space.
Just two examples of this unique collection of talent are chief scientific officer Rosemary Mazanet, MD, PhD, and chief data officer Guy Hussussian. Mazanet is a Dana Farber-trained haematologist/oncologist who has been instrumental in ensuring that all Columbia Care products are optimised for the best possible patient outcomes. Her strong scientific and medical credentials have played a critical role as Columbia Care sets a high bar globally for its pharmaceutical-quality, consistent medical cannabis products.
Hussussian has more than 20 years of experience in engineering, IT and operations, including at IBM where he started the infrastructure engineering and cloud operations group responsible for IBM-Aspera high-speed transfer services. In his role as chief data officer, Hussussian works with Columbia Care’s expansive registry of medical cannabis patient-reported outcomes. Guided by his deep expertise, Columbia Care is leveraging this dataset to not only understand how specific Columbia Care formulations help targeted patient populations but also use this knowledge to develop the most effective, innovative products in the industry.
With both a chief scientific officer and a chief data officer Columbia Care is demonstrating its enduring commitment to product quality, innovation and patient experience.
Scientific Advisory Board
The Columbia Care SAB, led by Mazanet, has deep expertise in oncology, data science and healthcare provider settings. Bustamante is invaluable in accelerating Columbia Care’s data analytics program and solidifying relationships with global technology leaders. Stephen Bonner, former president and CEO of Cancer Treatment Centers of America, is leading the process of integrating our products and services into traditional and specialty healthcare provider settings, including insurance formularies and healthcare insurance plans.
Market-specific advisory boards
Part of Columbia Care’s success can be attributed to its efforts to understand and fully integrate itself into the local culture in which it operates. It aims to be a local partner in each market, and this is achieved with the help of its meticulously designed market-specific advisory boards.
For example, the formation of Columbia Care’s German Advisory Board was done to ensure the company understood the needs and expectations of German stakeholders (policymakers, researchers, providers, institutions and patients). The German Advisory Board for Columbia Care Deutschland GmbH is the model the company intends to use in every European market where it needs to maintain and embrace regional and local expertise.
These advisory boards are going to be essential for establishing local leadership credentials and ensuring that Columbia Care always uses generally accepted best practices, rather than simply taking the US approach and applying it globally.
Global presence
Columbia Care is extending its thought leadership and presence beyond the US initially through its license in Malta. Upon completion of the final regulatory steps, Columbia Care will be able to import, export, cultivate, process and distribute medical cannabis in Malta, allowing it to access markets globally where federal medical cannabis programmes exist. Consistent with its existing operations in the US, Columbia Care expects to launch with its portfolio of pharmaceutical-quality cannabinoid-based medicines throughout Europe and the rest of the world.
Operations in Malta are expected to be best in class, including a GMP-certified and ISO-certified research and manufacturing facility where it will produce the full suite of its proprietary pharmaceutical-quality medical cannabis products, including ClaraCeed, TheraCeed and EleCeed, in tablet, vaporisation oil, tincture, suppository and topical formulations. Columbia Care will also grow a targeted selection of medical-grade flower strains derived from its proprietary genetic bank containing over 250 phenotypes.
This announcement is Columbia Care’s first response to enquiries from patients, medical leaders, policymakers and institutions throughout Europe, South America and Oceania to provide access to its patented portfolio of pharmaceutical-quality, dose-metered cannabinoid-based medicines.
In the UK, Columbia Care works with Families 4 Access to help provide data and educational materials for legal cannabis use as it is implemented. In addition, Columbia Care is a participant in the Centre for Medicinal Cannabis.
Columbia Care’s efforts specifically in Germany highlight its proven model for establishing and maintaining a meaningful presence in new markets. Recruiting and maintaining partnerships with regional leaders and experts ensures meeting the needs and expectations of regional policymakers, researchers, healthcare providers, institutions and patients. For example, Columbia Care’s partnership with MöllerPharma in Germany unites decades of regional GMP-compliant production of medicinal plant-based extractions, isolates and tinctures with Columbia Care’s proprietary and trusted brands, capital resources, data and scale.
Summary
Columbia Care is a nearly 100% vertically integrated company that cultivates the highest quality medical cannabis products in the industry. The company’s superior ability to manufacture and dispense on a global scale, build international brands and continuously uphold strict quality standards has enabled it to become one of the market leaders in the United States, and Columbia Care is leveraging this position to grow into the one of the most trusted medical cannabis companies in the world.
Since its inception six years ago, Columbia Care has used its unique abilities, leveraging the collective expertise of its executive team, to grow into an industry leader, setting the standard for pharmaceutical-quality, consistent cannabis-based medicines. With its vision of solving some of the world’s most challenging unmet medical needs and mission of improving lives through product innovation, research and patient experience, Columbia Care is not only a world-class cannabis business, but a healthcare company that is truly working to transform patient lives with its unparalleled cannabis-based products and ongoing commitment to rigorous scientific research.
References
- National Academies of Sciences, Engineering, and Medicine. 2017. The health effects of cannabis and cannabinoids: Current state of evidence and recommendations for research. Washington, DC: The National Academies Press.
- Framework for FDA’s Real-World Evidence Program. December 2018. www.fda.gov
Columbia Care LLC
info@col-care.com
https://www.col-care.com/
Please note, this article will appear in issue 8 of Health Europa Quarterly, which is available to read now.


















Hello.This post was really motivating, particularly because
I was looking for thoughts on this matter last Thursday.