Led by Professor Hans Bisgaard, the COPSAC research unit focuses on phenotyping children and their environmental exposures to study asthma and other chronic inflammatory diseases
COPSAC (Copenhagen Prospective Studies on Asthma in Childhood) aims at unravelling complex disease mechanisms in a translational research process to improve prevention and treatment of asthma and other inflammatory diseases.
Asthma is a common inflammatory disease of the respiratory system affecting 10% of schoolchildren with approximately 300 million sufferers worldwide. It causes attacks of breathlessness and coughing and can require hospitalisation and lifelong medication.
The prevalence of the condition has more than doubled in recent decades, but it remains poorly understood how the exposome – the totality of exposures from the modern environment – and its interactions with the genome have initiated such a surge in cases.
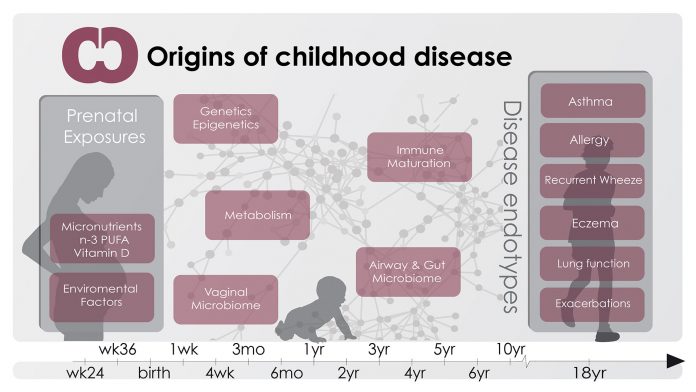
Recent investigations into the origins of asthma have focused on identifying how early life exposures to factors such as dietary components and the human microbiome – the microbiota colonising and living in symbiosis with humans – may be responsible for a deviant immune maturation, causing asthma and other chronic inflammatory conditions that have increased in prevalence in parallel with asthma.
It is important that chronic conditions such as asthma are not accepted as being normative traits of some individuals, and it is our belief that closely monitored longitudinal mother-child cohort studies are necessary to obtain the required knowledge to promote and foster change and develop new preventative and treatment strategies.
Longitudinal mother-child cohort studies

In order to test the hypothesis that asthma is caused by exposures in pre- and neonatal life, the Danish COPSAC team, led by Professor Hans Bisgaard, has carried out two decades of research based on longitudinal cohort studies. The initial programme – COPSAC2000 – involves 411 children born to asthmatic mothers between 1998 and 2001.
The children have been monitored at the COPSAC research unit from birth and every six months for seven years, and again at 13 years for clinical outcomes such as asthma, eczema, allergies, lung function and bronchial responsiveness. They attend additional acute care visits for any symptoms related to the airways or the skin.
This cohort includes a randomised controlled trial (RCT) on the effect of early intervention with inhaled corticosteroid from the onset of asthma symptoms, documenting that the natural history of asthma cannot be altered with this approach. The COPSAC2000 participants are currently returning for thorough clinical assessments at age 18.
The successive cohort – COPSAC2010 – is more wide-reaching, following 700 unselected pregnant women and their children, monitoring general development and signs of clinical disease, so far during the first six years of life.
Pregnant women were recruited during 2008-2010 by a monthly surveillance of reimbursement to general practitioners for the mandatory pregnancy visit. The deep clinical phenotyping performed in the COPSAC longitudinal studies has assessed early patterns of exposure and physiological reactions through a multitude of tests.
The children are examined using foetal ultrasound; dual energy X-ray absorptiometry (DXA) scans; physical examination; tympanometry; lung-, immune-, cognitive- and motor-development functioning tests; blood sampling; microbiological swabbing; home environmental measures (nicotine and house dust); and extensive bio-banking.
The wide range of techniques all serve the aim of capturing the unique circumstances of each individual case, accurately assessing overall health and identifying environmental exposures prior to birth and during early childhood before the development of symptoms.
Complementary to the mother-child cohorts, COPSAC takes advantage of the high quality Danish health registries, providing prospective assessments of hospitalisation and drug prescriptions in all Danish children. The health registries have been used to validate findings from the COPSAC birth cohorts by providing less-specific disease phenotypes but increased power due to large numbers.
Furthermore, COPSAC has established the COPSACsevere cohort of approximately 1,200 children with recurrent severe asthma exacerbations. In COPSACsevere, the registry data on hospitalisations was combined with the Danish national biobank. The discovery of a novel asthma gene (CDHR3) and unprecedented strong effects for known asthma genes demonstrated the strength of analysing extreme phenotypes.
Endotyping
It is increasingly recognised that conditions such as asthma are syndromes consisting of several disparate subtypes caused by separate disease mechanisms (endotypes), and it is through the identification of these that medical practice in the future may move away from symptom-driven disease classification and treatment, and towards mechanism-based individualised treatment and prevention – termed ‘precision medicine’.
It is a driving hypothesis at COPSAC that the accurate assessment of disease presentation and intermediate traits is a prerequisite for identifying such endotypes. Collecting clinical data describing the disease continuum from birth and onwards has the potential to unlock previously unknown mechanisms of disease, thereby enabling the discovery of different treatment responses and providing insights into genetic susceptibility, immune deviation and environmental influences.
Asthma begins before birth
A unique feature of the COPSAC2000 cohort is the measurement of lung function and bronchial reactivity at age four weeks. We were able to demonstrate that children developing bronchiolitis and asthma later in childhood had pre-existing lung function deficits and hyper-reactive airways as neonates. These results emphasise that future studies of asthma should focus on prenatal exposures and disease mechanisms in order to understand and prevent disease.
Diet

Research conducted in the COPSAC cohorts include the influence of maternal diet on the immune maturation of the developing child and the potential to prevent disease.
Lifestyle changes in Western societies have resulted in a decreasing intake of micronutrients such as vitamin D and n-3 Polyunsaturated fatty acid (PUFA), and it has been hypothesised that this could cause lifestyle-related ‘welfare’ illnesses, such as asthma. The causality behind such lifestyle-dependent associations can only be proven by RCTs, but previous trials of supplementation with micronutrients in pregnancy have been too small and have shown ambiguous results.
The COPSAC2010 study therefore included nested double-blind RCTs of high-dose vitamin D and n-3 PUFA or placebo supplementations for mothers in the third trimester of pregnancy.
The supplementation with n-3 PUFA during pregnancy reduced the risk of asthma in the offspring by 30%. The protective effect was strongest in children of mothers with low n-3 PUFA levels before the intervention and/or with a fatty acid desaturase (FADS) genotype associated with low n-3 PUFA levels.
In these groups PUFA supplementation caused a 54% reduction in childhood asthma.
Furthermore, the supplementation with n-3 PUFA also led to a reduced risk of lower respiratory tract infections in childhood.
The high-dose vitamin D supplementation during pregnancy suggested a 25% reduced risk of asthma. This result was not statistically significant in COPSAC2010 alone, but a subsequent combined analysis of the COPSAC2010 vitamin D trial and an American trial of similar design showed consistent results and a statistically significant protective effect of vitamin D supplementation.
These findings promise simple and safe prevention strategies that may significantly relieve the large burden of persistent wheezing and asthma in children.
Furthermore, they provide evidence that the origin of asthma begins in utero. Future COPSAC studies will focus on understanding the mechanisms behind these preventions.
The human microbiome
In recent years, an increasing awareness of our microbiome as a potential disease modifier has arisen with studies of the complex microbial communities in the gut, airways, skin and all other compartments of the body.
These microbial communities are known to play an important modulatory role in the immune maturation of the child from birth. The COPSAC team demonstrated that pathogenic bacteria a neonate’s airways is a risk factor for later asthma, while increased faecal bacterial diversity in early life seems to protect against later development of allergy.
The earliest microbial composition of the child is dramatically affected by the mode of delivery. The COPSAC research group recently showed that delivery by caesarean section was associated with asthma and, interestingly, we further demonstrated, using the national registries, that it is also a risk factor for inflammatory bowel disease, juvenile arthritis and other chronic inflammatory diseases.
COPSAC is now conducting studies to understand specific early-life microbial maturation patterns associated with later development of disease. This includes high-throughput sequencing of microbial samples collected longitudinally from pregnancy throughout childhood.
Such studies may be pivotal for future targeted, efficient microbiota manipulation for children at risk.
Genes and gene-environment interaction
Genetic factors play an important role in the development of asthma. Twin studies indicate that asthma has a heritability of 70-90%, and genetics is therefore one important approach to understand disease mechanisms. We have performed genetic studies of the COPSAC children, and have revealed important genetic risk variants for asthma and eczema.
Examples of this are the filaggrin mutation (FLG) – which causes an epithelial barrier defect resulting in susceptibility to environmental factors and eczema – and DENND1B, controlling the adaptive immune response. In addition, a genome-wide association study in the COPSACsevere cohort identified CDHR3 as a novel susceptibility locus for asthma with severe exacerbations, and this gene was later shown to function as a rhinovirus C receptor.
These gene discoveries demonstrate the high value of precise clinical phenotyping. Furthermore, combining genetic information with the longitudinal clinical assessments in the COPSAC cohorts has added to our understanding of disease endotypes.
For example, it was demonstrated that children with FLG mutation had a different clinical asthma presentation than children with the risk variant in the strongest known asthma locus on chromosome 17q21.
In spite of these and other gene discoveries, only a small part of the estimated heritability is explained, a phenomenon termed ‘missing heritability’. One possible explanation is that we need to account for interaction between genetic susceptibility and the environment.
For example, it was demonstrated in COPSAC2000 that children carrying the FLG mutation particularly were at increased risk of developing eczema if they were exposed to a cat from birth. Similarly, children with the risk allele at the 17q21 locus seemed more susceptible to rhinovirus infections.
One possible mechanism by which environmental factors can affect our genes is through epigenetics. Epigenetics is defined as heritable changes in gene expression without changes in the underlying DNA sequence. Epigenetic changes may explain a part of the ‘missing heritability’ as well as many of the characteristics of asthma, including long-term programming of disease from prenatal exposures and changes in disease status over the life course.
This will be the focus in future COPSAC studies by the assessment of epigenetic variations in blood and respiratory epithelium.
Divergent immune maturation
Asthma and other chronic inflammatory diseases are thought to emerge when the immune maturation process takes a deviant path, resulting in increased inflammatory responses to environmental factors.
To study the topical immune distortion of the neonatal airways in children developing asthma, we introduced a novel non-invasive method to sample mucosal lining fluid from the airways with filter papers. With this methodology, we are able to measure unstimulated levels of a range of immune mediators representing different immune pathways.
These neonatal airway immune fingerprints confirmed the influence of the early life milieu in the origins of asthma. For example, COPSAC has shown that children colonised with certain pathogenic airway bacteria as neonates have an up-regulated local airway immune response.
To study whether these bacteria actually influence the pathogenesis of asthma, we applied functional immune assays stimulating stored peripheral blood mononuclear cells (PBMC), collected at age six months, with the same pathogenic airway bacteria. This demonstrated that children developing asthma and children suffering from recurrent lower respiratory tract infections have an aberrant immune response at six months, highlighting that an early life immune deviation and insufficient response to the exposome may be a key element in the inception of asthma.
Our findings show that the immune response is determined early in pre- and perinatal life and is of importance for asthma pathogenesis. We showed that risk factors for asthma, such as reduced neonatal lung function and bacterial airway colonisation, are associated with systemic low-grade inflammation.
Furthermore, children with asthma and allergies by age seven years had increased inflammatory markers.
Interestingly, such inflammatory markers are also seen in many other chronic inflammatory disorders, suggesting that early life immune perturbation may be a common trait of both asthma and other chronic inflammatory disorders. In addition, we have assessed topical immune profiles from children with acute respiratory symptoms in order to understand immune mechanisms related to asthma exacerbations.
The canary in the coal mine
Through a comprehensive phenotypic characterisation of inflammatory immune diseases with a focus on asthma, the COPSAC team has successfully begun to chart the complex ‘Nature versus Nurture’ debate in terms of gene-environment interactions, and how this results in different disease endotypes.
The studies have successfully shown that there are key components of the environment, such as dietary deficiencies and the human microbiome that seem to play a causal role in the development of asthma, and potentially other inflammatory diseases, through interaction with predisposing factors in mother and child.
The COPSAC studies thereby highlight potential ways to prevent asthma through dietary supplementations and manipulation of the microbiome, and suggest novel methods of precision prevention through genetic markers.
COPSAC has until now focused on asthma, eczema and allergy, which are the earliest and most prevalent chronic inflammatory diseases occurring in childhood. These might be considered ‘the canary in the coal mine’, providing a disease model with implications for a wider range of other chronic inflammatory diseases such as obesity, arthritis, diabetes and inflammatory bowel disease.
In the future, the classification of specific mechanisms for inflammatory immune diseases will enable better understanding of the susceptibility to disease, which will help with identifying at-risk children and ultimately improve the treatment and prevention of disease.
Key references
1. Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, et al. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N Engl J Med. 2016 Dec 29; 375(26): 2530-9
2. Chawes BL, Bønnelykke K, Stokholm J, Vissing NH, Bjarnadóttir E, et al. Effect of Vitamin D3 Supplementation During Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. JAMA. 2016 Jan 26;315(4): 353-61
3. Bønnelykke K, Sleiman P, Nielsen K, Kreiner-Møller E, Mercader JM, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014 Jan; 46(1): 51-5
4. Sleiman PM, Flory J, Imielinski M, Bradfield JP, Annaiah K, et al. Variants of DENND1B associated with asthma in children. N Engl J Med. 2010 Jan 7; 362(1): 36-44
5. Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007 Oct 11; 357(15): 1487-95
6. Bisgaard H, Hermansen MN, Loland L, Halkjaer LB, Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med. 2006 May 11; 354(19): 1998-2005