Since 2004 the main interest of Jeanette Erdmann´s scientific work has been the identification of the genetic causes of coronary heart disease (CHD) and myocardial infarction (MI).
In the last ten years, genome-wide association studies (GWAS) have given completely new insights into the pathogenesis of CHD and MI. Today, more than 160 genetic variants associated with an increased risk of CHD/MI have been identified. Each of these variants only marginally increases the risk of disease, but overall these variants can identify patients with a multiple increased risk (1).
The identification of risk variants has also contributed to the identification of previously unknown signalling pathways that play an important role in pathogenesis. In addition to validating the importance of lipid metabolism, GWAS provided decisive evidence that the NO signalling pathway or inflammation are of great importance in pathogenesis. Moreover, Erdmann´s team systematically investigate families with a high incidence of CHD. In 2013 they were able to elucidate the genetic cause of the frequent occurrence of MI in a large German family and identify a new signalling pathway that plays an important role in the development of MI (2).
In their research, translational approaches have a growing relevance. In recent years, together with European and American colleagues, Erdmann´s team have been able to identify genetic variants associated with protection against diseases, including CHD/MI. Based on such genetic findings, several new, promising therapeutic targets are currently in experimental development. At the Institute of Cardiogenetics, for example, researchers are currently working on therapeutic approaches with the aim of influencing ADAMTS7, one of the many CHD/MI risk genes.
Research is going private
In addition to the research interests outlined above, six years ago – after decades of odyssey in search of a diagnosis for her muscle weakness known since birth – Erdmann was able to diagnose her own collagen VI myopathy using methods of modern genetics. Erdmann´s research agenda and the implications of her congenital muscle disease were described in detail in a feature of the European Heart Journal in summer 2018 (3).
By exome sequencing she succeeded in identifying a pathogenic mutation (p.G283E) in the Col6A2 gene (4). In retrospect, she shows all symptoms of an intermediate form of collagen VI myopathy, such as generalised muscle weakness, severe scarring (keloids), relatively slow progression (ability to walk maintained until the sixth decade of life) and severe impairment of the respiratory muscles (nocturnal non-invasive ventilation for more than 20 years).
Collagen VI myopathies, previously also known as Bethlem or Ullrich myopathy, are caused by mutations in the three collagen VI genes Col6A1, Col6A2 and Col6A3. The disease is one of more than 8,000 rare diseases with a frequency of 1-9/1,000,000. So far, no therapy for collagen VI myopathy is known.
Over the past five years, RNA-based therapy concepts have played an increasingly important role in various diseases. This is illustrated by the FDA approval of Nusinersen, an antisense oligonucleotide (ASO) for the treatment of spinal muscular atrophy (SMA). It is the first compound to treat SMA, a rare disease that is still the most common genetic cause of death in infants and young children. Other antisense drugs are under development – for example against Huntington’s disease or amyotrophic lateral sclerosis (ALS). New successes in the approval of RNA therapeutics are reported almost every month. A large number of clinical studies on the safety and efficacy of RNA therapeutics are also under development in the field of cardiovascular diseases.
RNA-based therapies attack even before the “blueprint” read from the DNA has been converted into a protein. In principle, this usually involves the suppression of mutated gene copies at the RNA level by means of so-called small interfering RNA (siRNA) or antisense oligonucleotides (ASO).
Also, in the case of collagen VI myopathy, suppression of the mutated allele should lead to therapeutic success. In the literature, first steps towards the establishment of RNA-based therapies can be found for individual mutations in the Col6A1 or Col6A3 gene. Since mutations in the Col6A2 gene are generally less common than mutations in the Col6A1 and Col6A3 genes, the current interest is more in the development of therapeutic approaches for the latter two genes.
Supported by the Dr.-Holger-Müller-Foundation (doctoral scholarship for Svenja Kohler) Erdmann and her team has started – together with Prof. Dr. Georg Sczakiel, Director of the Institute for Molecular Medicine at the University of Lübeck – to establish an RNA-based therapy concept for the mutation p.G283E in the COL6A2 gene in the sense of individualised medicine. The long-term goal is to generally establish the therapeutic approach for mutations in the Col6A1, Col6A2, and Col6A3 genes.
In order to investigate and optimise the general problems of the administration of RNA therapeutics, Erdmann participate in the COST Action DARTER (CA17103; Delivery of Antisense RNA Therapeutics).
First, based on her own skin fibroblasts, her team has established an assay to quantify the consequence of the present mutation on the extracellular matrix in vitro. In parallel, they have constructed ASO and siRNAs to suppress the mutated mRNA. The transfection efficiency as well as the sensitivity and specificity for selected ASO/siRNAs are promising.
In the coming months, the already selected and partially tested ASOs and siRNAs will be further optimised, and the “repair” of the extracellular matrix will be shown in vitro. The team is currently working on siRNAs that specifically attack p.G283E as well as on oligos that are located in the immediate vicinity of the mutation against common variants. The transfection efficiencies achieved so far are very promising.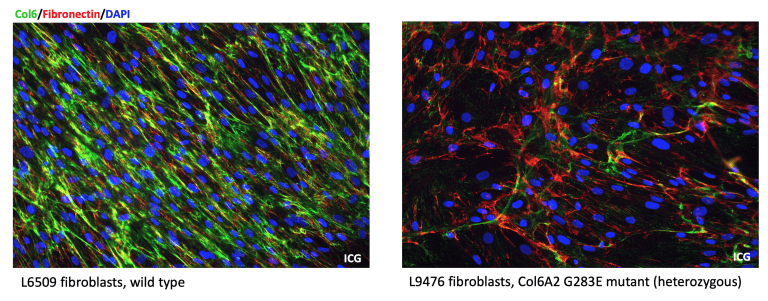
Figure 1: Representation of the extracellular matrix in the presence of the mutation p.G283E (right panel) in comparison with the wild type (left panel). The drastic reduction of collagen VI in the presence of the pathogenic mutation p.G283E becomes very clear in the right panel.
References
- Erdmann J, Kessler T, Munoz Venegas L, Schunkert H. A decade of genome-wide association studies for coronary artery disease: the challenges ahead. Cardiovascular research. 2018;114(9):1241-57.
- Erdmann J, Stark K, Esslinger UB, Rumpf PM, Koesling D, de Wit C, et al. Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature. 2013;504(7480):432-6.
- Ozkan J. Jeanette Erdmann PhD. Eur Heart J. 2018;39(22):2024-6.
- Erdmann J, Schunkert H. Forty-five years to diagnosis. Neuromuscular disorders : NMD. 2013;23(6):503-5.