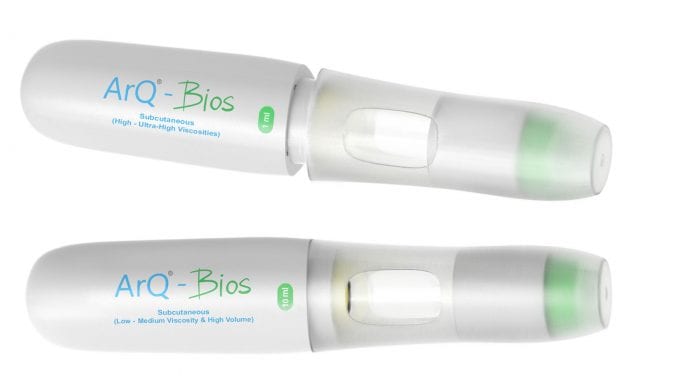
The development of biologic drugs and enabling a flexible autoinjector platform for the delivery of high viscosity or high-volume biologic formulations.
Recent advances in molecular biology have accelerated the development of a new class of therapeutics. Biologics offer more targeted therapies and fewer side effects compared to the small-molecule drugs which came before. Due to poor absorption through the digestive tract, as well as susceptibility to damage from digestive enzymes and acid, biologics cannot be administered orally. Because of their molecular size (e.g. IgG: 150kDa; aspirin: 0.18kDa), biologic drugs tend to have higher mass doses. These large therapeutic doses favour the injected rather than inhaled or nasal route. In the next decade injectable drugs are expected to be the largest growth segment in drug delivery. (1) Most of this growth is driven by the continued development of new biologic drugs and biosimilars.
As the industry moves towards self-administration to reduce healthcare costs and improve convenience for patients, there is a compelling need for simple-to-use, low-cost disposable devices for domestic use.
Biologic drugs are prone to gelling and aggregation at higher concentrations, limiting the ability to concentrate them to volumes traditionally available in autoinjectors (1ml and 2.25ml prefilled syringes) (2) as this requires an increase in concentration from 1g/l to around 200g/l (as in the case of Cimzia). This has resulted in many biologic therapies being released as intravenous infusions which require administration by trained healthcare professionals. One solution is the reconstitution of a formulation from a previously lyophilised material (costly and runs the risk of freezing).
Outside of production, higher concentrations of biologics create issues for injectability by leading to high viscosity and non-Newtonian properties. (3) The alternative approach is therefore a more dilute large volume injection. Injecting a large volume requires either a high flow rate, which can cause pain for the patient, or longer injection duration, which can be uncomfortable and difficult for the patient as it requires them to maintain position and activation force. This has given rise to devices such as bolus injectors, (4) which can deliver gradual subcutaneous injections of over 20ml. These lower concentrations are amenable to cheaper filtration-based concentration and sterilisation techniques and have less associated risk of loss of efficacy caused by lyophilisation, opening the door to a greater variety of biologic molecules.
There may be a more convenient option. The advent of recombinant hyaluronidase adjuvants such as Halozyme’s Enhanze offer the ability to increase the dispersion and absorption of injected drugs. They work by cleaving the linkages between N-acetylglucosamine and glucuronate to catalyse the degradation of hyaluronic acid and increase the permeability of the subcutaneous tissue: this potentially allows an autoinjector to deliver a large fluid dose in a relatively short time without creating discomfort for the patient. As the licensing of the technology by companies such as Roche, Baxalta, Pfizer, Janssen, AbbVie, Lilly, Bristol-Myers Squibb, Alexion and Argenx5 leads to broad industry acceptance, autoinjectors may return as the preferred vehicle when launching large volume therapeutics.
Understanding Formulation Delivery
Unforeseen difficulties in delivery – including settling, aggregation or greater shear thinning tendency – may only become apparent when injecting with a needle and syringe (e.g. settling, aggregation or greater shear thinning tendency). To aid optimisation of Oval’s containers for a new application, Oval has developed a proprietary Injection Characterisation System, which allows performance criteria to be rapidly translated into tangible design options, such as the geometry, materials and needle bore specifications at an early stage.
Injection Characterisation System (ICS)
Oval’s proprietary Injection Characterisation System (ICS) overcomes issues associated with traditional rheological methods by performing measurements in situ. The system resembles an autoinjector with the addition of a range of sensors to provide data on the injection process. This provides a vast range of information about the formulation behaviour during delivery. Changes in the formulation arising from temperature, shipping and ageing can be detected.
Describing non-Newtonian fluids
Many biologic formulations exhibit shear dependant Power Law behaviour at higher concentrations. (3) Power law fluids demonstrate a time independent log-log relationship between shear (du/dr ) and viscosity (μeff) described by:
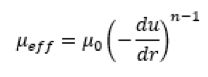
Where n* is the flow behaviour index which dictates how much the fluid viscosity (μ0) reduces under shear.
Oval have created a mathematical model for the injection time of these types of fluid in an autoinjector:
![]()
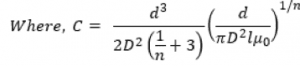
Where L is the delivery stroke (mm), F0 is the initial spring force, k is spring rate, D is the container internal bore and d is the needle bore. The result is a slowing down of the injection progress as spring force diminishes at the end of injection.
Autoinjector Monte Carlo simulations
Using this model in a Monte Carlo simulation generates predicted delivery time for millions of simulated devices through random sampling of the input variables. Each input variable is described by an appropriate statistical distribution generated from predicted process capability.
Using these techniques and a detailed understanding of difficult to deliver formulation, Oval have developed a high-power single-use autoinjector (ArQ-Bios) which offers pharmaceutical companies the ability to choose between high viscosity or high volume dose options in the same device. This allows some flexibility in decision making during formulation development and allows it to occur in parallel with device design without risk to the device architecture and therefore time to market.
A flexible platform for large volume or high viscosity therapies
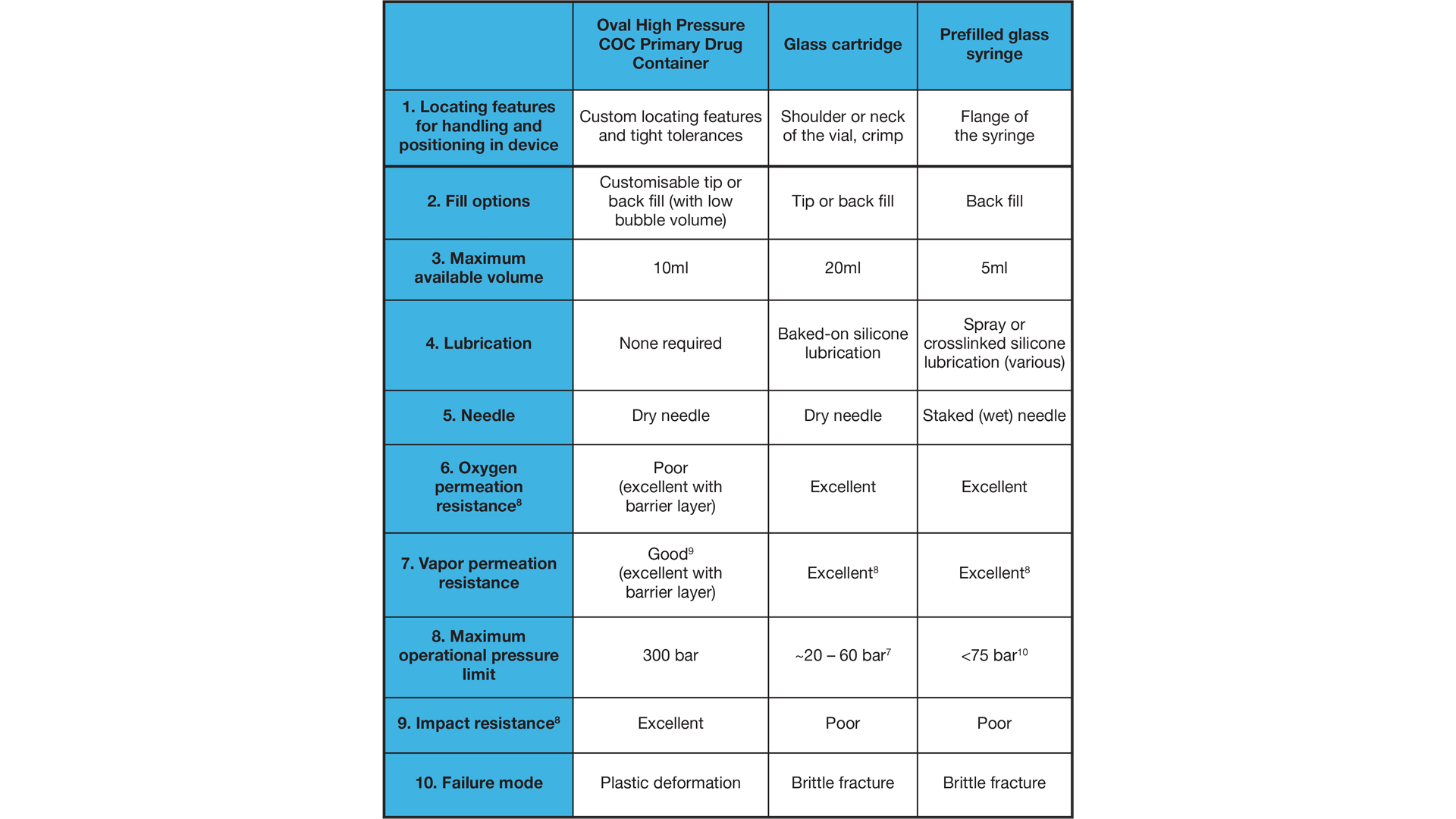
Oval’s ArQ Bios platform is built around a high-pressure cyclic olefin copolymer (COC) Primary Drug Container (PDC). The PDC can safely tolerate significantly higher pressure than glass, allowing stronger springs to be used in the AI design. This enables the device to generate higher pressures than other market offerings. Additional coatings are available to adjust the drug contact material and improve barrier properties. (6)
A route forward
Oval have developed a system to offer flexibility in early-stage development by allowing for the delivery of either high viscosity or high volume biologic formulations. The system is lubricant-free and able to achieve performance not possible in traditional glass primary packaging. Establishing partnerships between biopharmaceutical companies with primary packaging manufacturers early in product development may help create unique solutions to the challenge of delivering better solutions to patients and carers.
References
1) Otto R., et al., (2014). Rapid growth in biopharma: challenges and opportunities. McKinsey & Company Insights.
2) Pathak, J. A., et al., (2013). Do clustering monoclonal antibody solutions really have a concentration dependence of viscosity?. Biophysical journal, 104(4), 913-23.
3) Burckbuchler V., et al., (2010). Rheological and syringeability properties of highly concentrated human polyclonal immunoglobulin solutions. Eur J Pharm Biopharm. 76(3):351-6.
4) www.springboard.pro/bolus-injectors
6) www.sio2med.com/technology.html
7) www.us.schott.com/tubing/english/products/properties/burst-pressure.html
8) Forcinio, H., (2018). Selecting Primary Packaging for Parenterals. Pharmaceutical Technology, July, 42(7), p. 46–50.
9) www.us.schott.com/pharmaceutical_packaging/english/products/syringes/polymer_syringes.html
10) Wohland, A. et al., (2009). Encoding and Reading of Codes on Glass Containers for Pharmaceutical and Diagnostic Products. Die Pharmazeutische Industrie, 71(10), pp. 1770-1774.
This article is from issue 16 of Health Europa. Click here to get your free subscription today.


























