Lorna Rothery spoke to Professor Till Bachmann, an expert in molecular diagnostics and Chair of the JPIAMR’s SAB, about how diagnostics can inform optimal antimicrobial therapy.
The use of rapid diagnostic tests to aid appropriate antimicrobial therapy can have a huge impact on patient outcomes and clinical awareness. Most importantly, access to diagnostic tools is not equal across the board with low-and-middle-income countries often lacking the necessary infrastructure to support, for example, timely antimicrobial susceptibility testing of bacteria. Furthermore, while there is a significant drive to fill the antibiotic abyss, improving equitable access to current and new diagnostic tools is equally important, and requires a multisectoral approach.
To discuss the integral role of diagnostics in the context of antimicrobial resistance, as well as some of the latest initiatives helping to drive progress in this area, Lorna Rothery spoke to the Joint Programming Initiative on Antimicrobial Resistance (JPIAMR) SAB Chair and expert on diagnostics, Professor Till Bachmann.
How integral are rapid diagnostic tests in aiding antimicrobial therapy and reducing the spread of AMR?
The choice of antimicrobial therapy today is generally empirical, without diagnostics of the disease-causing pathogen. Therapies are predominantly based on clinical judgement and guidelines which are informed by local epidemiology.
Diagnostics should be a much stronger integrated part of antimicrobial therapy decision making but unfortunately, they are not. That is why we are aiming to improve the situation.
What types of rapid diagnostic tools are currently being used to guide appropriate treatment?
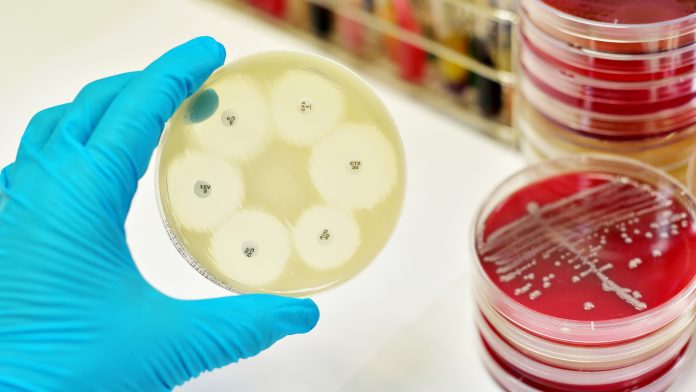
The vast number of microbial diagnostics are growth-based using standard Petri dishes. The 100-year-plus technology involves taking clinical samples and cultivating bacteria which are present on agar plates so that we can analyse them. Depending on the assay, and the specific method, you can identify the bacteria as well as antibiotic susceptibility. There are all sorts of different variations of those growth-based assays but none of them is truly rapid because it takes time for the bacteria to grow.
We have seen a very strong increase in MALDI-TOF – mass spectrometry-based identification of the species or the strain – to identify microbial pathogens isolated from the sample or clinical specimen.
There are more and more technologies coming that use molecular ways of identifying specific aspects of bacterial infections. There are also tools which detect biomarkers of infection, and these are increasingly used but could be used more.
There are disparities in the use of diagnostic tools in low and high-income countries, as well as in different healthcare settings. In the hospital, it is very easy to get your sample to the central lab where a growth-based assay is done. However, if you are in primary care, the sample often needs to be transported from primary care into the hospital, or a suitable facility, where they can do growth-based assays.
Transporting samples and then growing the bacteria so that it can be identified is very time-consuming and often means clinicians do not have the information to hand to help inform their clinical decisions. Many areas within low and middle-income countries just do not have the facilities to grow the bacteria in the first place, which is a huge problem.
Would you say the COVID-19 pandemic has helped to accelerate the development and adoption of new rapid diagnostic tools for AMR?
It was very interesting to see how the COVID-19 pandemic spurred activities in the field of diagnostics. Of course, there was the attractive opportunity of more available grant funding, but people also wanted to do their bit and contribute their specific expertise to try and find solutions. Many of the AMR or bacterial diagnostic groups and companies endeavoured to develop COVID diagnostics.
Nevertheless, the vast majority of tests used at the end for COVID were lateral flow tests and PCRs. It turned out that COVID could be detected with these standard technologies. It is a little disappointing when one considers the technologies used, and those that have been developed for AMR and COVID; there are all sorts of high-end rapid tests and yet we are using old technologies.
At the same time, lateral flow tests and PCRs delivered, and the implementation of these tests has been possible. Moreover, tests were needed quickly in huge numbers. These could be provided in time using existing manufacturing technologies and up-scaled facilities.
Many people who developed technologies for COVID are now shifting their focus back to AMR because, of course, the COVID pandemic has evolved and the demand for testing has changed. But, increased capacities have been built and activities to support the pandemic preparedness efforts including data sharing and surveillance have built on pre-pandemic efforts and are now benefitting the field of AMR.
COVID enhanced the acceptance of antimicrobial diagnostics for policymakers and the general community but also shifted some focus away from AMR.
What are some of the key barriers to the development, implementation, and use of rapid diagnostics in the field of human and also veterinary medicine?
I coordinated two major joint programming initiatives on antimicrobial resistance and funded networks to address the barriers to the development and implementation of rapid diagnostic tests. There were around 50 partners involved each and we welcomed academics, clinicians, and industrial colleagues into these networks to really look at these barriers from all possible angles.
There are many factors to consider, a key one being that people very often underestimate the cost requirements for developing a rapid diagnostic test. The nature of antimicrobial resistance is problematic; while COVID is essentially one pathogen and has a relatively accessible nucleic acid and so a very clear target, with AMR, it is a huge moving target. You have many different pathogens, many different diseases and many different mechanisms of resistance which are variable among the pathogens. You also have different sectors which impact AMR development – human, environmental and animal. All those contribute to a multifaceted problem.
In terms of developing diagnostic solutions, you have to understand your science and your use case. In general, there is a clear disconnect between the technology developers, researchers, the people funding the research, and the end user.
We identified one major factor was the absence of suitable target product profiles which specify exactly which type of characteristics a test should have, and how these characteristics should look. For example, how fast the test needs to be, where the test is carried out, the disease it is for, and the actionable result.

Very often with AMR, we think we need to identify antimicrobial susceptibility – which antibiotic you could use – but a key question sometimes comes much earlier which is, ‘should I use antibiotics or not?’ For upper respiratory tract infections, for example, a very simple test would be required and would have a very different specification to a test for more severe cases, let’s say for bloodstream infection. Diversity of required test specifications is the key challenge and the biggest barrier to development and implementation.
Payment is of course connected to reimbursement schemes for the healthcare system that the patient is in, the question of who pays for the test is intimately linked with health economic considerations. If you have an insurance-based system, you need to prove that the use of the diagnostic can give some benefit, and here is another limitation, we do not have enough data to support this. We need to show and demonstrate the health economic benefit, and the patient benefit of using rapid diagnostic tests in more studies. There are projects running, including the IMI project VALUE-Dx, that is doing this for rapid diagnostic tests for acute respiratory tract infections in community healthcare settings. This is what we need for other disease indications, and other settings.
The IMI project focuses on Europe, and we need to have similar projects done in low and middle-income countries. The evidence will lead to user acceptance; if the clinician or the prescriber does not trust the result, does not see the benefit for the patient, or does not have the decision pathways to use that diagnostic information they will not use it. A test needs to be simple and fit into clinical pathways otherwise it will lead to reduced acceptance and adoption. We need to work more with the end user and those who make decisions for diagnostics.
I come from a biosensor background and moved into diagnostics and am originally very much technology-driven, but we are moving more and more towards a user-driven approach, going out into the community to better understand who is using the tests and how they need to be presented. Behavioural change is a huge driver for the adoption of diagnostics.
Teaching and training were other barriers we identified through the JPIAMR networks. People need to be trained to understand the benefits of diagnostics and how to use them. The COVID-19 pandemic certainly helped with this; nobody questioned the place of diagnostics in leading some intervention decisions. The patient can also voice the expectation that they are tested before they are given a drug.
Can you tell me about the AMR-RDT working group and what it has managed to achieve since being established?
The working group was funded through the JPIAMR with industrial, academic, clinical partners, and other colleagues, so it was a multi-stakeholder working group. It was then succeeded by AMR Dx Global, another working group picking up the teaching and training aspects. We published, for example, a roadmap on test development, raising awareness and providing input into what we think are the barriers and how we should be addressing them. From there, multiple activities followed: JPIAMR launched a call for Diagnostics and Surveillance Networks in April 2022. (See: https://www.jpiamr.eu/calls/network-call-2022/) and is also in the process of updating the JPIAMR Strategic Research and Innovation Agenda, contributing to the development of the candidate One Health AMR Partnership.
Outcomes from this working group, together with all the other networks which we have been involved with make for a very interesting phase in the AMR field. A lot of attention has been focused on One Health – looking at humans, animals, and the environment – and seeing the interaction of transmission. People understand this much more now following COVID – with AMR and the use of antibiotics which can be released into the environment, the use of them in agriculture, and then issues on the human side – people understand that link. It is clear that more research needs to be done in the field, but it is a really interesting time in terms of the multiple activities going on to tackle AMR.
Are there any recent developments in the field of diagnostics that you would like to highlight?
I have been coordinating an Indo-UK project called DOSA (Diagnostics for One Health and User Driven Solutions for AMR) which looks at how diagnostics can help use antimicrobial therapy to tackle AMR in One Health settings in the community in India. In human health, difficulty in seeking medical care means that people have to live with conditions or buy antibiotics over the counter, meaning people’s conditions can worsen.
We developed diagnostic solutions for that, in a co-design approach with members of the community and community healthcare, and these diagnostics are currently entering the deployment phase for validation. We are moving away from very centralised testing into point-of-care and potentially home testing. The big caveat with this is we do not want everyone to test for everything and anything at home, and then demand antibiotics, we are very wary that we are not driving up antibiotic consumption. The aim is early detection and proper healthcare management and awareness.
I am also on the advisory panel for the Longitude Prize which was set up by the UK Government and comprises an £8 million award for a team of innovators who develop a point–of–care diagnostic. It garnered a lot of attention for AMR diagnostics and antimicrobial therapy and is hoped to continue to do so. The winner needs to demonstrate that they have reached a level of maturity in terms of manufacturing and clinical performance. They need to demonstrate a set of criteria and from what I see, many applicants are on a very good track overall.
There will be one winner at the end, but a lot of teams have benefitted from the overall support through feedback from the panel of experts and the Longitude Prize team. £8m is not enough to develop a diagnostic tool, unfortunately, but it certainly helps.
Finally, I recommend – with all the important focus on the development of new antibiotics – that we must not lose sight of the other interventions to tackle AMR. Diagnostics need to play a big part in this.
Professor Till Bachmann
Chairman of the scientific board
Joint Programming Initiative on Antimicrobial Resistance
https://www.jpiamr.eu/
https://se.linkedin.com/company/jpiamr
https://www.facebook.com/JPIAMR/
https://twitter.com/jpia
https://www.youtube.com/channel/UC9TXetkVWUbCgM4fn2iNZ9A
This article is from issue 22 of Health Europa Quarterly. Click here to get your free subscription today.

























