The University Hospital Münster
The University Hospital Münster, Germany, uses peripheral and central stimulation strategies to treat post-stroke dysphagia. The oropharyngeal swallow involves a rapid, highly co-ordinated set of neuromuscular actions beginning with lip closure and terminating with the opening of the upper esophageal sphincter. The central co-ordination of this complex sensorimotor task uses a widespread network of cortical, subcortical, and brainstem structures. Among the many diseases and disorders affecting the central swallowing network, stroke is by far the most common. In acute stroke, more than 50% of patients are reported to experience swallowing problems. Due to the complications of post-stroke dysphagia (PSD), including malnutrition, dehydration and aspiration pneumonia, length of hospital stay is nearly doubled in affected patients and the long-term outcome is generally worse with a twofold rise in mortality, higher levels of dependency, and the need for institutional care compared to non-dysphagic stroke patients. With regards to the additional burden to the healthcare system, PSD significantly increases medical expenses with yearly costs being about €3,900 higher per person than for a patient without dysphagia, even when controlling for age, comorbidities, and proportion of time alive.
New treatment options for PSD on the horizon?
Although both from the epidemiological and the individual patient perspective, the impact of PSD is obviously considerable and a matter of concern, there is hardly any well-established medical treatment for this debilitating condition. Currently, most guidelines for clinical management of PSD focus on the prevention of complications, while any natural recovery processes may take place.
Thus, patients are fed via a nasogastric tube, receive modified diets and thickened fluids, or compensatory manoeuvres like the chin tuck or the supraglottic swallow are used.
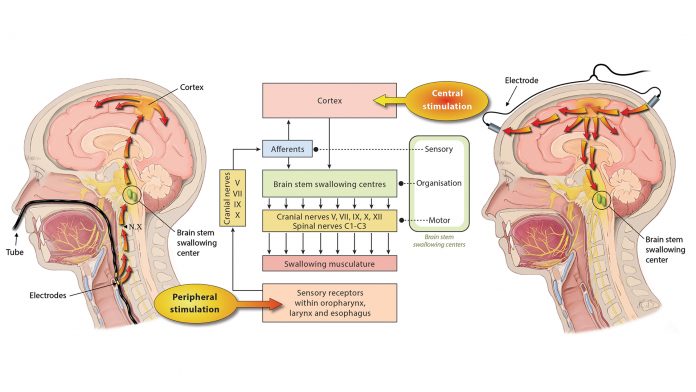
However, since the knowledge on neural repair mechanisms has increased substantially over recent years, different types of neurostimulation treatments have grown in popularity and are currently being researched in this field. As shown in Fig. 1, these modalities may generally be differentiated in central and peripheral stimulation strategies. The common underlying mode of action for those types of stimulation is to enhance functional recovery by promoting plastic changes of the cortical swallowing network.
Central Stimulation
Both repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) have been used to directly target the swallowing cortex. Single pulse TMS applied over the motor cortex is able to induce the motor-evoked potentials of the pharyngeal muscles, and 5-Hz rTMS targeting the same cortical regions has been shown to increase excitability of the corticobulbar pharyngeal projections. tDCS in turn applies a tonic electrical stimulation that modulates the resting membrane potential of neurons and thereby leads to an alteration of spontaneous discharge rates.
So far, and following promising results in neurophysiological and proof-of-principle studies, both techniques have been used in smaller, single centre trials recruiting patients suffering from PSD. As has been suggested by recent meta-analyses, the application of rTMS or tDCS was related to dysphagia improvement both immediately after treatment and during long-term follow-up.
Peripheral Stimulation
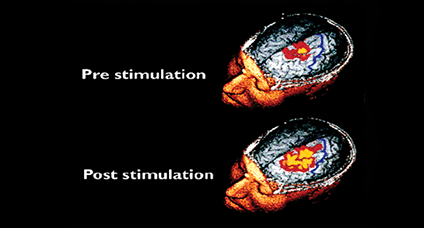
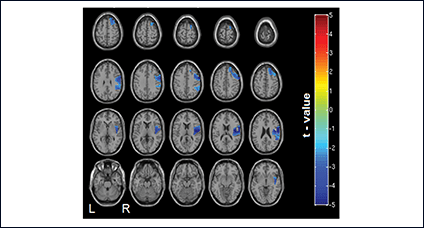
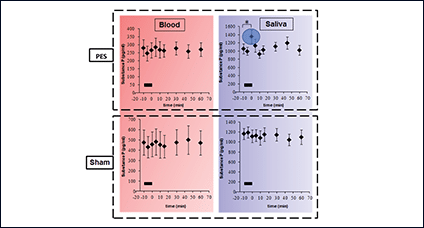
Using catheter-mounted electrodes, pharyngeal electrical stimulation (PES) applies an electrical stimulus to the posterior pharyngeal wall. Exploring the basic physiological principles of this approach, several studies have shown that PES enlarges the pharyngeal motor map (Fig. 2), modulates the cortical swallowing network (Fig. 3) and increases concentrations of specific neurotransmitters (Fig. 4). PES has been adopted in several clinical studies recruiting different types of stroke patients. While results have been heterogeneous, further studies are being conducted and others expected to follow.
Taken together, different neurostimulation treatment modalities addressing the unresolved issue of PSD are carefully scrutinised. Although there is a strong need for more high-level scientific evidence, a fundamental paradigm shift with regards to the therapeutic approach targeting PSD is likely to occur during the next decade.


References
- Dziewas, R et al. ‘Recognizing the Importance of Dysphagia: Stumbling Blocks and Stepping Stones in the Twenty-First Century’. Dysphagia 2017. 32(1): p. 78-82