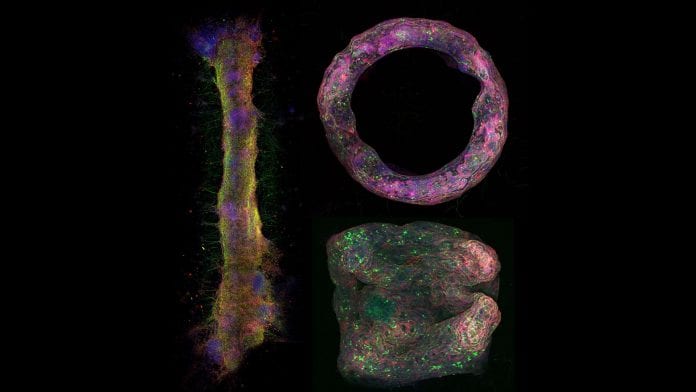
In a first of its kind move, researchers have formed 3-dimensional tissue consisting of neurons.
Researchers at the University of Illinois at Urbana-Champaign have successfully used stem cells to engineer living biohybrid nerve tissue to develop 3D imaging models of neural networks.
The researchers hope it will help them to gain a better understanding of how the brain and these networks work.
The first author, Gelson Pagan-Diaz-Diaz, a graduate student in the Department of Bioengineering at the Grainger College of Engineering, likens the produced tissue to a computer processing unit, which provided the basic principle to today’s supercomputer.
He said: “Being able to form 3-dimensional tissue consisting of neurons can give us the ability to develop tissue models for drug screening or processing units for biological computers.”
3D imaging model
The brain is challenging to study in an actual person. Being able to understand how these networks develop using a 3D model outside the body promises to give researchers a new tool to better understand how it works.
These models will be able to help understand how abnormalities form, for example, what gives rise to diseases such as Alzheimer’s.
The team was able to give 3D geometry to the living tissue made of neurons which optogenetics, so they could be activated with blue light. These tissues could be used to study complex behaviours that happen in the brain and how these tissues react with new drugs being developed.
Bashir said: “If we can control how these neurons communicate with each other, if we can train them using optogenetics, if we can program them, then we can potentially use to perform engineering functions.
“In the future, our hope is that by being able to design these neural tissue, we can begin to realize biological processing units and biological computers, similar to the brain.”
The project was funded through an NSF Science and Technology Center EBICS (Emergent Behaviors of Integrated Cellular Systems) and published this month in the Proceedings of the National Academy of Sciences.
Neural tissue mimics
In this study, the team developed neural tissue mimics that can form different shapes. The team used hydrogels and fibrin to make millimeter to centimeter scale structures that doesn’t have rigid scaffolds and can be moulded into a number of desired shapes.
Pagan-Diaz explained: “It’s a bundle of hundreds to thousands of microns of cells that contains a lot of populations with a genetic makeup similar to in vivo tissues.
“As we continue develop these bio-fabrication methods, we should be able to capture a lot of the phenomena that happens in vivo. Once we can prove that, we will be able to mimic the morphology that we see in the brain. Once we show that the tissue engineered outside the body is similar to the tissue in the body, then we can then fabricate them over and over again.”
Pagan-Diaz continued: “Being able to fabricate these tissue mimics outside the body allows us to characterise and study their electrical activity in great detail, the broad set of design rules due to the 3D structure and shapes gives you a lot more experimental freedom and open up new avenues of research in neuroscience, medicine, and engineering applications.”
























