Speaking to Health Europa, Professor Michael Lisanti, MD-PhD, FRSA discusses the potential role of antibiotics in preventing the recurrence of cancer.
How would you cure cancer? It was this question, asked to their then eight-year-old daughter Camilla, that inspired husband and wife Michael P Lisanti and Federica Sotgia, both professors at the University of Salford, UK, to explore the use of antibiotics as an anticancer therapy – a journey that now looks set to transform not only the management of cancer but also the development of new drugs.
How did the innocent idea of a child lead to a research project of such revolutionary potential? Lisanti tells all in conversation with Health Europa.
The reverse Warburg effect
The first breakthrough came at the Kimmel Cancer Center in Philadelphia, US, where Lisanti had been investigating the protein caveolin-1, a topic on which he is now considered a leading expert. He tells Health Europa that he had been working alongside a pathologist to better understand the protein’s role in cancer, and specifically its presence in the epithelia, but hit a stumbling block when he discovered that the “presence of caveolin in breast cancer cells has absolutely no prognostic value whatsoever”. He had been chasing the wrong lead.
Undeterred, Lisanti turned his attention instead to the stroma, i.e. the connective tissue of the tumour. This time he was more successful, arriving at the realisation that a loss of caveolin in the stroma is a predictor of recurrence, metastasis, drug resistance and death. Now that he had identified the diagnostic, Lisanti set about uncovering the mechanism behind it.
“The loss of caveolin was actually indicative of autophagy in the fibroblasts, which was being induced by oxidative stress. This was causing the fibroblasts to eat themselves in order to make food to feed the cancer,” he explains. “Essentially, then, what we had discovered was the mechanism underlying why people with cancer lose weight; the cancer is eating them alive.”
Based on these findings, Lisanti proposed an entirely new metabolic model for cancer – the
so-called ‘reverse Warburg effect’. Lisanti describes this model as a “radical and, at the time, a heretical alternative” to that made popular by Nobel laureate Otto Heinrich Warburg, who posited that cancer cells produce most of their energy via a process called glycolysis, in which glucose is broken down independently of oxygen.
Lisanti was the first to suggest that the opposite is actually true: “Aerobic glycolysis in the connective tissue makes food to feed the cancer cells – and that food is actually mitochondrial fuel.”
The mitochondria as a target
This breakthrough, which is now widely accepted, put energy transfer and mitochondria at the forefront of efforts to fight cancer, but Lisanti initially struggled to secure the funding necessary to take his research further.
He tells Health Europa: “By 2012, I had moved to the UK and, by 2014, myself and my colleagues had a good handle on another controversial topic: cancer stem cells (CSCs). Nobody knew exactly what was fuelling these cells, but, after purifying them and then performing proteomics, we realised that both the mitochondrial mass and the energy were increased in CSCs.
“At that time, I tried to get funding to make and test drugs that would inhibit the mitochondria, but, unfortunately, in industry and in academia, people wrongly assume that you can’t safely target the mitochondria. They think that doing so will kill the patient.
“Obviously, there is some validity to that, but everything is a dose response and, as long as we’re careful, it can be done.”
How to cure cancer
It was while considering how exactly to achieve this that Lisanti received his next burst of inspiration – this time from an unlikely source.
“One night, after my wife and I had finished talking about homework, school and sports with our daughter, we got to talking about how we could cure cancer. When we asked Camilla how she would do it, she said she would just use an antibiotic like when she had a sore throat.”
What Lisanti didn’t know at that time was that mitochondria and bacteria have an evolutionary relationship dating back 1.45 billion years.
“A long, long time ago, bacteria snuck into cells and, eventually, over a process of years and years, they became mitochondria,” he explains.
What that means in practice is that mitochondria can be effectively targeted with antibiotics, just as Camilla suggested, including those already approved and on the market.
Lisanti and Sotgia took this idea into the lab, testing the effects of common, FDA-approved antibiotics like azithromycin and doxycycline on tumour-sphere formation in 12 different cell lines.
To their surprise, the antibiotics eradicated CSCs in all eight cancer types examined, including breast, ductal carcinoma in situ, prostate, lung, ovarian, pancreatic, and even melanoma and glioblastoma.
“That was a big shock,” Lisanti admits, “because we had been thinking about how to target mitochondria safely and without side effects, and it turned out to be obvious in hindsight.”
The result was a 2015 paper in Oncotarget, on which Camilla – who is now 12 – was named as a co-author, that suggested treating cancer as though it is an infectious disease, using antibiotics whose safety profiles have long been established.
In focus: doxycycline
Encouraged by their early findings, Lisanti and Sotgia began to focus their research efforts on doxycycline, a commonly prescribed antibiotic that first came into commercial use in 1967 and is today used to treat such conditions as acne, periodontitis, rosacea and chlamydia. Lisanti explains that doxycycline was selected as the best candidate for anticancer therapy given its 22-hour half-life, 100% absorption rate and high safety profile (it can be taken for six months at a time without major side effects).
In a recent Phase II window trial, Lisanti and Sotgia tested the effects of doxycycline on nine cancer patients at the University Hospital in Pisa, Italy, each of whom received the orally administered antibiotic for two weeks just prior to surgery.
Lisanti explains: “We took a biopsy at the surgery stage and compared it to one we had taken at diagnosis. We were particularly interested in looking at CSC markers because it was those that would give us an indication of whether or not the doxycycline was actually targeting the cancer stem cells – we looked at two in particular, CD44 and ALDH1 – both known biomarkers of cancer stemness.”
The results were remarkable. On average, CSCs were reduced by 40%, and close to 90% of the patients responded positively to the doxycycline – something unheard of in most clinical trials or current chemotherapies.
The trial targeted only breast cancer patients, but Lisanti is excited about extending the investigation to include other cancer types and a much larger number of patients.
He is now looking ahead to doxycycline one day being administered to cancer patients as an add on to the current standard of care, chemotherapy – should a Phase III trial prove successful.
Already, Lisanti and Sotgia have attracted a lot of interest from physicians keen to test the discovery on their own patients, and a number of people have reported benefitting from the findings.
“Obviously there is a lot more work to be done,” Lisanti says, “but in controlled settings, doxycycline seems to be working as predicted, which is hugely promising.”
Especially exciting is the low cost of the antibiotic, which comes in at just 10-12 pence (~€0.12-0.14) per patient per day.
“There are no real drugs that can target the CSCs, but here we have one that is inexpensive, readily available, safe and – best of all – it has a huge impact. This could reduce the cost of cancer therapy tremendously, which could be particularly significant in low-income countries, where financial constraints make current therapies more challenging.”
Lisanti acknowledges that this trial is not the first to uncover a link between antibiotics and cancer (previously published works have focused on the use of doxycycline against cancer-associated infections, in MALT lymphoma and non-small cell lung cancer). However, Lisanti’s and Sotgia’s trial is the first to specifically and deliberately target the cancer stem cells, and he is hopeful that its success will inspire similar studies in the future.
“There was some early excitement about metformin – a diabetes drug – as an anticancer treatment, and that led to hundreds of metformin trials. Ours is the first doxycycline trial, and I would predict that once it becomes more well-known, others will follow suit.”
Opportunities in drug development
Beyond its implications for cancer therapy, Lisanti’s and Sotgia’s work also holds enormous promise in drug development, something which is especially notable given the current climate of antimicrobial resistance.
Lisanti tells Health Europa: “We took an antibiotic that had been designed to kill bacteria and used it instead to kill mitochondria in cancer cells. But the reverse is also true: if we can make a drug to kill mitochondria in cancer cells, then that drug would also be an antibiotic and we could use it to kill bacteria – not just gram-negative or gram-positive bacteria but also pathogenic yeast and even antibiotic-resistant bacteria like MRSA.”
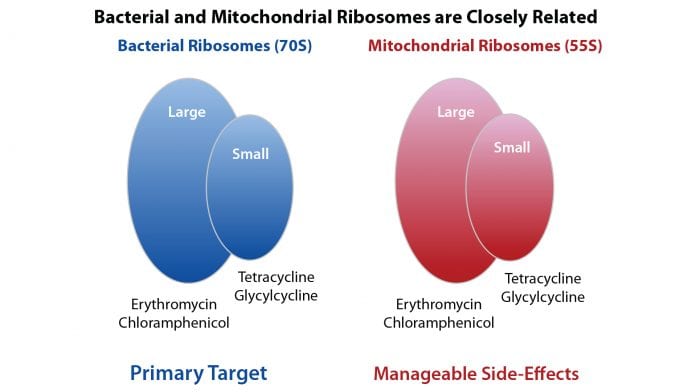
Lisanti and Sotgia have named this new class of antibiotics mitoriboscins – so-called because they target the mitochondrial ribosomes in cancer stem cells.
“At the moment we’re just scratching the surface, but essentially what we have created is a new method of making antibiotics that doesn’t even use bacteria. That approach could one day be used to target the big problem of antibiotic resistance; before, there was no systematic way of discovering an antibiotic, but now we’ve been able to show proof of principle that all you need is to find something that binds to the target, to the mitochondrial ribosome.
“Now that the cat is out of the bag, those drugs can be optimised by medicinal chemists and our approach could be used by pharmaceutical companies to develop more powerful chemotherapies and antibiotics.”
Looking to the future
Lisanti and Sotgia now plan to explore the use of doxycycline in combination with either chemotherapy or other FDA-approved drugs. They have already found various combinations in culture that are more effective than doxycycline alone, and Lisanti is excited about what he describes as a “new era” in drug discovery.
“At the same time as making new drugs, we can also optimise or repurpose old drugs for cancer – and, because we don’t need to do Phase I trials, the speed at which we can do that could accelerate FDA approval by between ten and 15 years, not to mention save billions of pounds. We know doxycycline is effective against CSCs by itself, but if we could make it more potent by using combination therapies, then we could bring it to the clinic even faster.”
In the meantime, Lisanti is keen to see a shift in industry away from bulk cancer cells as the focus of drug production and towards CSCs. He’d also like to see industry overcome its reluctance to embrace mitochondria as a target.
“A number of antibiotics already target the mitochondria as an off-target side effect; we’re proposing that this side effect be repurposed as the therapeutic effect, which is really just a question of semantics,” he explains.
“I’m hoping that other people will pick up the ball on this, because we don’t have the resources to do everything by ourselves. Obviously we have collaborators, but it would be great if some other pharma companies joined us, as well – there is a huge unmet need here and a huge clinical opportunity.
“Most cancer patients don’t die from the primary tumour, which is what current therapies are designed for – in fact, in surgery they take the tumour out. What most patients die from is the recurrence and the metastasis. Finding a way to target that has proven to be a big stumbling block, but now we know that we can use ordinary antibiotics to deplete or control the CSC population.
“I really believe this could turn into a blockbuster drug, if not in terms of making money then certainly in terms of curing cancer.”
Indeed, what Lisanti and Sotgia have discovered could not only dramatically reduce the cost and length of oncology drug development but also one day transform cancer into a manageable chronic disease – proof that the innocent ideas of children really can be revolutionary.
This research has been supported by the Foxpoint Foundation (Canada), the Healthy Life Foundation (UK), the Pisa Science Foundation (Italy) and Lunella Biotech, Inc. (Canada).
About Michael P Lisanti
Professor Michael P Lisanti currently serves as the chair of translational medicine at the University of Salford School of Environment & Life Sciences, UK. His research focuses on, inter alia, epithelial-stromal interactions and metabolic-symbiosis in cancer, and anti-ageing therapies in the context of age-related diseases such as diabetes and dementia.
Lisanti began his education at New York University, US, graduating magna cum laude in chemistry (1985), before completing an MD-PhD in cell biology and genetics at Cornell University Medical College, US (1992). In 1992, he moved to MIT, US, where he worked alongside Nobel laureate David Baltimore and renowned cell biologist Harvey Lodish as a Whitehead Institute fellow (1992-96).
His career has since taken him to the Albert Einstein College of Medicine, US (1997-2006), the Kimmel Cancer Center, US (2006-12), and the University of Manchester, UK (2012-16), where he served as the Muriel Edith Rickman chair of breast oncology, director of the Breakthrough Breast Cancer and the Breast Cancer Now Research Units, and founder and director of the Manchester Centre for Cellular Metabolism.
Lisanti has contributed to 551 publications in peer-reviewed journals and been cited more than 80,000 times. A list of his works can be found at https://www.ncbi.nlm.nih.gov/pubmed/?term=lisanti+mp
About Federica Sotgia
Professor Federica Sotgia currently serves as chair in cancer biology and ageing at the University of Salford School of Environment & Life Sciences, UK, where she focuses on, inter alia, the role of the tumour microenvironment in cancer and the metabolic requirements of tumour-initiating cells.
Sotgia graduated magna cum laude with an MS in biological sciences (1996) from the University of Genova, Italy, where she later completed a PhD in medical genetics (2001). She moved to the Albert Einstein College of Medicine, US, in 1998, originally as a visiting student and then postdoctoral fellow, and was appointed an instructor in 2002.
Sotgia has since worked as an assistant professor at the Kimmel Cancer Center, US (2006-12), a senior lecturer at the University of Manchester, UK (2012-16), and a reader in biomedical science at the University of Salford (2016-17).
She has contributed to 195 publications in peer-reviewed journals and been cited
upwards of 22,000 times. A list of her works can be found at www.ncbi.nlm.nih.gov/pubmed/?term=sotgia+f
Future pharma developments: Lunella Biotech, Inc.
To accelerate new anticancer and antimicrobial drug discovery, as well as anti-ageing drug development, Sotgia and Lisanti have been actively collaborating with an Angel investor, resulting in the creation of a new innovative small pharma company, called Lunella Biotech, Inc., founded and based in Canada. Lunella Biotech is named after Camilla Luna Lisanti, the little girl that was the catalyst for this new adventure in drug discovery. This was the wish of the Angel investor and his dedicated team.
Lunella Biotech, Inc., has already filed new patents to protect the intellectual property arising from this new approach to medical therapy, by targeting the mitochondria. This will ensure the implementation and funding of new clinical trials, to test the safety and efficacy of the new pharmaceuticals that Sotgia and Lisanti develop, to target cancer stem cells, ageing senescent cells and microbial resistance in bacteria.
Lisanti says: “We envision a brighter future, when ageing, cancer and infectious disease can all be effectively managed and perhaps even prevented, alleviating the fear, pain and suffering that these serious conditions cause in patients and their families.”
Since large pharmaceutical companies have not been tremendously successful at making new drugs that target these broad therapeutic areas, Lisanti explains: “We are taking a more assertive, do-it-yourself approach, to drug discovery.”
If Lisanti and Sotgia can identify FDA-approved drugs to treat these conditions, then they can also build safer, more effective and newer molecular approaches that have been optimised to fill these currently unmet medical needs, to directly benefit patients.
Find out more
- C Scatena et al.: Doxycycline, an inhibitor of mitochondrial biogenesis, effectively reduces cancer stem cells (CSCs) in early breast cancer patients: A clinical pilot study. Frontiers in Oncology (2018)
- F Sotgia et al.: A mitochondrial based oncology platform for targeting cancer stem cells (CSCs): MITO-ONC-RX. Cell Cycle (2018)
- E M De Francesco et al.: Cancer stem cells (CSCs): metabolic strategies for their identification and eradication. Biochemical Journal (2018)
- U E Martinez-Outschoorn et al.: Cancer metabolism: a therapeutic perspective. Nature Reviews Clinical Oncology (2017)
- M Fiorillo et al.: Mitochondrial “power” drives tamoxifen resistance. Oncotarget (2017)
- M Peiris-Pagès et al.: Cancer stem cell metabolism. Breast Cancer Research (2016)
- R Lamb et al.: Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: treating cancer like an infectious disease. Oncotarget (2015)
- U E Martinez-Outschoorn et al.: Caveolae and signalling in cancer. Nature Reviews Cancer (2015)
Professor Michael P Lisanti, MD-PhD, FRSA
Chair in Translational Medicine
School of Environment & Life Sciences
University of Salford
+44 (0)1612 950 240
M.P.Lisanti@salford.ac.uk
www.salford.ac.uk/environment-life-sciences
This article will appear in issue 7 of Health Europa Quarterly, which will be published in November 2018.

























