
ProPharma Group talk us through the process of creating a trial using chewing gum containing THC.
ProPharma Group has a global network of experts to support you in development of drug product, entering clinical studies and help you to get approval for a medical cannabis product in your country.
This article will provide an example of how we successfully supported a client with an idea of using chewing gums with THC for medicinal purposes.
Introduction to cannabis
The medicinal cannabis industry is rapidly maturing.
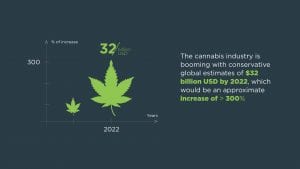
The cultivation of Cannabis sativa plants has increased substantially over the last few years and extraction techniques have been developed.
Therefore, THC and CBD are now readily available both as the pure compounds and as full spectrum cannabis extract in which most of the cannabinoids that have identified in the cannabis sativa plant are present including terpenes.
Simultaneously, several clinical studies have been conducted to understand whether cannabinoids can treat conditions or help alleviate symptoms. In only a few cases have they resulted in successful outcomes and approval was obtained from the health authorities.
Well-known examples are: Marinol (in the US, chemically synthesised THC to treat loss of appetite in people with AIDS and nausea in cancer patients), Sativex (1:1 ratio of full spectrum THC and CBD to improve symptoms of spasticity in MS patients ) and epidyolex (a CBD oral solution to treat rare but serious forms of childhood onset epilepsy).
The latter product surprisingly acts – at least partly – through de-activating a number of CYP-enzymes in the liver responsible for metabolising Clobazam, which is often a co prescribed drug in those patients.
The primary metabolite of Clobazam is active as well and its further metabolism is delayed due to the inhibition of a CYP-enzyme. The built-up of the primary metabolite may well be partly responsible for the favourable outcome. Further research is ongoing to unravel the mechanism of action.
Other studies in a range of conditions have not shown significant effect compared to placebo; at least not statistically significant. Many factors may have contributed to this such as the study was not significantly powered (i.e. the number of patients was not sufficient to demonstrate efficacy over placebo), or the type of the medicinal product used (i.e. pure cannabinoids or mixtures, such as full spectrum cannabis extracts with or without terpenes).
On top of that, many different types of route of administration have been used: inhalers for pulmonary administration, oromucosal and oral administration, as well as topical formulations. Another question that may arise is, have the inhaler devices and vaporisers been studied to a level that accurate and consistent dose delivery is guaranteed? All these variables make it hard to compare one study to another and to find out why there is anything more to treatment with cannabinoids than the aforementioned products.
Whether extracts or purified cannabinoids are used during these studies, there are several possibilities to administer active substances to patients; each with their own pros and cons.
When working with medicinal cannabis, it is important to start with the patient in mind and define requirements that will treat a patient in the most comfortable / appropriate way possible.
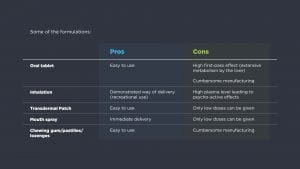
There are quite a few patient groups strongly advocating the use of THC, CBD or mixtures for treatment of various conditions such as PTSS, ADHD and pain. They often report that they have found in cannabinoids an effective treatment for their ailment after a long search for the best combination of active substance, administration route and delivery device. Can we consider cannabinoids as possible personalised medicines in which for every patient a unique combination is needed? To continue the studies to discover more about treatment with cannabinoids, it is important to be able to navigate the field with an expert to support the processes.
We can only progress the use of cannabinoids as a drug through sound, science-based research using selected patient groups and employing standardised medicine. We are a consultancy company offering support for the pharmaceutical industry in the areas of life cycle management, regulatory affairs, pharmacovigilance and medical information.
We have supported many cannabis pharmaceutical companies in building quality systems, participation in tender procedures, import of medical cannabis from Canada and other non-European countries into Europe and support in filing CTAs and INDs for clinical studies using cannabis. In the last couple of years, we have also been involved in the development of a medicinal cannabis chewing gum.
Let me walk you through this adventurous journey.
Problem statement
The client is a virtual company lacking deep knowledge of developing a product towards clinical studies. They wanted to develop a formulation for oromucosal administration of THC or CBD or combination of both. A chewing gum formulation was chosen as the active substance would be administered in the buccal/oromucosal area thereby avoiding the oral route.
The oral route is known to deliver only low amounts of THC due to a first pass effect i.e. clearance of THC by the liver, which results in a bioavailability through the oral route of only 15%. It was speculated that mastication (chewing) would also have a positive effect on pain one of the possible indications. The use of other modes of oromucosal delivery were excluded as those would not require mastications. On this basis, ProPharma Group was asked to develop an overall development plan including formulation, regulatory and clinical strategies.
How did we approach this project?
Target product profile
The approach of ProPharma Group in developing a product is to start with the target product profile. It in fact defines the future labelling of the product and thereby helps focusing the development efforts. It is an important starting document and will be adapted during development based on the findings.
We defined the following aspects for the chewing gum:
- Product description: bio-comparable to existing marketed products in the field such as Marinol and Sativex. Striving for bio-equivalency was considered too daring as the formulation is so different from the oral and sprayed oromucosal formulations. There was also a wish to have higher dosages of CBD to treat PTSS (Post-Traumatic Stress Syndrome);
- Formulation: a gum of two grams containing up to 20 mg of THC and up to 400 mg of CBD;
- Release: preferably slowly but within five to 10 minutes;
- Stability: at least two years; and
- Markets: primarily US but also Europe.
Project team
After deciding on the TPP, a dedicated project team was set up to address all the activities needed to progress to the first clinical study which would be a PK/PD-study with the chewing gum containing THC. ProPharma Group has a network of over 1,100 in-house and external subject matter experts on formulation and analytical as well as on quality and regulatory aspects and clinical.
Complemented with project management services, we can support all activities in the quality and regulatory field needed to bring a product to clinical studies and beyond. As an independent partner, ProPharma Group can support you in navigating and managing all parties you need to involve such as regulatory bodies, manufacturing partners and contract research labs. On top of that we have a database with trusted vendors. External input was required to address the needs of this project. We defined four work streams necessary to support the development of this product.
- CMC (chemistry, manufacturing and controls ): consisting of experts in the field of extracting the plant and producing purified API’s (active pharmaceutical products) and formulation development as well as manufacturing exerts capable of carrying out the production of both the API and the chewing gum;
- QA (quality): consisting of experts who built a quality management system from scratch, covering both CMC and GCP aspects of the project. This team was also responsible for obtaining a manufacturing licence as well as the controlled substance licences to support the import and export of cannabinoid products as well as the storage of these products at CMO/CRO’s;
- RA (regulatory affairs): consisting of experts in the field who could support the development of the product throughout its journey; from developing the regulatory strategy to scientific advice at regulatory bodies as well as compiling and filing dossiers to support clinical study applications; and
- Clinical: consisting of experts who were responsible for drafting the clinical protocols and participant facing documents and were also in charge of executing the clinical study in healthy volunteers.
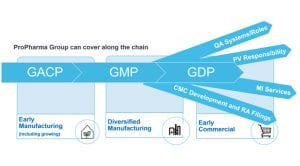
Each work stream was led by a project lead who represented his or her specialty within the core project team. The team was completed with a sponsor representative and a Project Manager. The core team was mainly focused on overall planning and resolving of issues, rather than on the technical aspects of the project. Technical aspects were dealt within the sub-teams and issues were escalated if these could impact the timelines, budget or compromise the desired outcome of the project. The team structure is depicted in the scheme below.
The team of those experts ran the daily operations of the sub teams. One example of such a sub-team is also given in the picture below.
The journey
CMC
With the goal set, the team composition secured, and the project plan written, all the elements were present to start the journey of developing a chewing gum containing cannabinoids.
Initial experiments defined the extraction procedure as well as the decarboxylation of both THC and CBD containing plant materials. This is in reverse to what is regularly done in this industry; first decarboxylation of THC and CBD while it is still present as the acid in the dried buds. This method has the advantage that via an acid/base extraction, terpenes can easily be removed and thus the purified cannabinoids can be obtained by usual purification methods.
Scaling up of this method proved to be possible and therefore we were soon able to obtain sufficient amounts of API (active pharmaceutical ingredient or drug substance) to fully start the development work of the chewing gum. Analytical methods for the API were developed including methods to demonstrate the absence of terpenes.
During development of the chewing gum, it soon became apparent that this was by no means an easy task. Both CBD and THC have a strongly lipophilic character and on top of that are unstable under oxygen and light conditions (except when CBD is in its crystalline form). It is well established that THC decomposes by a radical oxidative process. This precludes the simple mixing of ingredients to form a chewing gum as would typically be done in a tableting process. The lipophilic character of both the chewing gum and the APIs form a combination that will not easily separate and dissolve into the hydrophilic environment of saliva. On top of that, the unprotected APIs would not survive in such an oxygen-rich environment.
How did ProPharma Group meet this challenge from a development perspective? We selected three methods to protect the THC and/or CBD and to still be able to make it available for dissolution. A method based of self-emulsifying principles two distinctly different types of microspheres.
The formed intermediates would then need to be mixed with chewing gum base and further excipients to form a chewing gum. The selection of the best intermediate was done on the basis of stability, ability to release of API, prior IP and manufacturability. One of the microsphere options was chosen as the best way forward and scaled-up.
Tableting of the intermediate with chewing gum base and excipients proved quite uneventful. The only questions remained at which ratio of intermediate versus chewing gum base, the chewing gum still has the texture of a chewing gum. By performing some chewing tests with different ratios of chewing gum and a placebo intermediate product (the microsphere) a preferred ratio could be established which still provided the texture of a chewing gum.
The chewing gum would lose its texture and disintegrate rapidly. With that knowledge, it was clear that the amount of THC or CBD in the gum should be limited and the delivery of hundreds of milligrams of CBD into the chewing gum would not be possible in this way.
The analytical methods were developed and validated and in particular the method to test the release of API from the chewing gum. A chewing gum tester as per European Pharmacopoeia was used to demonstrate that release from such a complex matrix is possible and this method was also validated according to ICH-guidelines.
GMP production of both the API and the drug product went as planned and the material was now ready to start a first clinical trial to establish the PK/PD-profile.
Quality assurance
The customer assigned all QA activities to ProPharma Group. The following activities were included:
• Setting up a quality management system (QMS), a site master file (SMF) and the standard operating procedures needed to work under GMP-conditions;
• Performing audits at the CMO/CRO’s to the sponsor such as the clinical site and the development and manufacturing sites. This included audits at the clinical CRO and the sites where the API and the drug product were manufactured as well as the transporter of the clinical trial material;
• Manufacturing licence was required and ProPharma performed the relevant submission and was responsible for the communication with the authorities during the inspection; and
• Applying for the controlled substance licence. Although not physically handled by the Client, the inspection indicated that the client should also apply for such a licence as they are the owner of the THC-containing product.
These activities were all successfully completed which opened another door to conduct the clinical study.
Regulatory affairs
As with the QA activities, the RA-activities were also assigned to ProPharma Group. First, scientific advice was requested from regulatory authorities to make sure that we were on the right track comparing the chewing gum formulation to existing cannabis containing products. This was done by providing a detailed briefing document to the authorities, which included mostly questions on the clinical development of the product as well as questions on CMC aspects such as the specification of the chewing gum.
During execution of the CMC development plan, reports that formed the basis for the quality part of the IMPD were written by Propharma Group. In addition, non-clinical and clinical parts of the Investigator’s Brochure (IB) were also prepared. The IMPD together with the Investigators Brochure, the clinical protocol and other supporting documentation form the clinical trial application which is the basis of the assessment by the Ethical Committee to grant approval to conduct the clinical study.
All three activities were delivered successfully and on time.
Clinical
The clinical study will be conducted by a clinical CRO. The CRO developed the clinical protocol and supporting plans and related documents around data management, safety reporting, lab assessments, monitoring, etc. The sponsor/client maintains overall responsibility for the conduct of the study. Competent authorities advised that GCP compliance should be supported by an external person to ensure no bias towards the results and possible issues during the study. ProPharma Group GCP expert will support this aspect of the project.
Work streams coming together
Now that all work streams have delivered their objectives, the scene is finally set for a beautiful ending. Currently, the research ethics committee is reviewing our responses to questions they had after their first review and it is likely that the clinical study will start in the next few months.
Conclusion
This example shows that ProPharma Group has a good track record in supporting the complex activities needed to bring a product into clinical studies in the area of cannabinoids.
We set up a project team consisting of four different expertise areas, populated those with relevant SMEs and saw the project through to the initial stages of the clinical study. Now we look forward to the results of this first study in healthy volunteers, which will show us the way to further develop this product.
Jan Zorgdrager, PhD
Principal Consultant
ProPharma Group
+31 (0) 6 15 070 348
Jan.Zorgdrager@propharmagroup.com
Tweet @ProPharmaGroup
www.ProPharmaGroup.com
This article appeared in the first issue of Medical Cannabis Network which was out in January. Click here to subscribe.


















