
ProPharma Group takes a look at medical cannabis regulation and compliance.
Jan Zorgdrager, PhD, and Marc Stegeman, MSc, Principal Consultants in ProPharma Group’s Life Sciences division, explore medical cannabis regulation and compliance.
Mention the word cannabis and the confusion starts: legal or illegal; nutraceutical or medicinal product; psychoactive or non-psychoactive; clinically significant or not. In this article, you will find out more about the current and future regulations and compliance standards regarding medical cannabis.
Definitions
The first thing to set straight is the name cannabis. This is not a specific type of plant or product but more an all-encompassing term used to describe a number of plants, materials and products. While there are over a hundred known active substances (cannabinoids) in cannabis with slightly different chemical structures, the two most common are THC and CBD, which are representative of the majority of endeavours in cannabinoid product development.
Regulations
Currently there are more than 50 countries, mainly in Europe and the Americas, where medicinal cannabis is either wholly legal or legal with certain restrictions. It is usually prescribed by a physician and provided by pharmacies. Pharmacies obtain their packaged product from wholesalers and those from growers of cannabis plants. Both the growers and the distributors require licences from state or municipal offices to be allowed to work with this plant material. To obtain such a licence, most regulatory agencies require applicants to demonstrate:
- Accountability of cannabis material;
- Specific processes for handling the material;
- Controls and accountability of growing process;
- Importing controls;
- Re-packing protocols;
- Storage controls;
- Distribution oversight; and
- Research and development.
Numerous producers have been successful in obtaining the proper licensing; however, it can be complicated to produce or distribute in multiple countries at the same time, because each country has its own set of regulations.
Quality standards
When producing, storing or distributing medicinal cannabis, national health authorities may require a product to comply with the standards described in the country’s good manufacturing practice (GMP) requirements, such as those outlined within EU Member States. This is managed by clearly defining specifications for possible quality risk factors like:
- The content of the product;
- Moisture levels;
- Potential contamination by foreign matter; and
- Microbiological concerns.
Interestingly, GMPs are not required for planting or seeding of cannabis plants. Instead, this typically requires good agricultural practice, which ensures a constant quality of the plant material and is often conducted in a secured greenhouse. GMP oversight usually starts at harvest of plant buds.
Extract or purified substance?
Increasingly, the cannabis industry’s focus is shifting from plant material such as terpene oils to the extraction and purification of cannabinoids. While the plant material does exert a beneficial effect and dried buds are easy to obtain and use, both through smoking and in brewing the buds into a tea, it comes as no surprise that standardising the use of cannabis to secure consistent dosing for patients is key; and it is certainly worthwhile to investigate more advanced ways of delivering cannabinoids. For instance, by presenting an extract or purified THC or CBD to patients increases clinicians’ control over the content. In this way, dosages become more accurate, opening possibilities to investigate the efficacy of treatment in clinical trials. A number of companies are already using purified THC and CBD – essentially pure active substances – in clinical studies aimed at obtaining marketing authorisation.
The drug product Sativex, for example, is manufactured by extracting the cannabinoids from the entire cannabis plant and formulating them into a spray. GW Pharma obtained marketing authorisation for Sativex in 25 countries outside the US for treatment of spasticity. The authorisation of Sativex is quite remarkable, as the extracts from the cannabis plant within it still contain a large number of other cannabinoids, as well as terpenes. Although we assume the medicinal effects of Sativex are due to its THC and CBD content, it is not clear which components are specifically responsible for the therapeutic effect.
Formulation
Whether extracts or purified cannabinoids are used, there are several potential methods of administering these active substances to patients; each with their own pros and cons. When working with medicinal cannabis, it is important to start with the patient in mind and define individual requirements in order to treat a patient in the most comfortable and appropriate way possible.
Some of the formulations are detailed below:
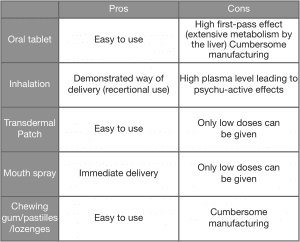
Stability
One aspect which needs to be taken into consideration when extracting and purifying THC is its stability, or rather its instability. Unlike CBD, THC is vulnerable to oxygen, light and possibly moisture; and it is readily decomposed by radical oxidation. This means that standard formulation techniques may not be sufficient to provide a stable drug product, requiring instead encapsulation techniques. Methods have been developed to protect the THC by encapsulation into a polymeric or a liposome matrix. Surprisingly, the dissolution in aqueous ethanol mixture also exerts a stabilising effect, as demonstrated by the Sativex spray developed by GW Pharma.
Clinical studies
As previously stated, a number of treatments have been approved around the world by health authorities. These are mainly in the area of pain reduction, emesis, nausea; and symptom reduction in MS patients. It is known that cannabinoids act on a multitude of different receptors in the body, either as agonists or antagonists; and that they could potentially interfere with disease mechanisms. Therefore, cannabinoids are thought to have the potential to treat or cure diseases such as cancer (see, for example, the excellent review conducted by Massi et al: www.ncbi.nlm.nih.gov/pmc/articles/PMC3579246/).
The future
So why haven’t we found more cures to date? Is it possible that the multiple compounds contained in cannabis material have hampered the progression of the use of cannabinoids in approved treatments? As indicated before, cannabis materials are challenging to define and characterise due to the number of cannabinoids and terpenes, along with the multitude of administration routes. Therefore, it is difficult to substantiate claims regarding the medicinal benefits of cannabis with clinical evidence. With THC and CBD now readily available as GMP material, the scientific community is more suitably equipped to perform clinical studies, hopefully resulting in approved drug products that treat patients safely and effectively.
From a business perspective, a key driver for success is increasingly becoming a swift and timely adaptation to an ever-changing regulatory landscape. For example, compliance with the EU’s novel foods regulation has become mandatory for producers operating within the bloc. To date, however, few applications have been granted licences under this regulation. The question therefore is not if, but when compliance with novel food regulations will be enforced. In addition, individual countries may have specific requirements. For example, in Ireland cold pressed CBD oil is not considered a novel food; whereas products containing CBD extracted using other techniques are. While EU regulations have created a tremendous harmonisation within the EU, there is still always a door which is not quite shut: country-specific requirements and exemptions.
Jan Zorgdrager
Principal Consultant at ProPharma
ProPharma Group
+31 (0) 71 524 40 00
+31 (0) 6 15 070 348 (Mobile)
Jan.Zorgdrager@propharmagroup.com
Tweet @ProPharmaGroup


















