
Zoono antimicrobial technology is bridging the gap between routine cleaning cycles by modifying skin and surfaces against bacteria and viruses.
It is well documented that surfaces can pose a significant threat as a source of microbiological contamination, both within the healthcare environment and beyond. It is particularly important to note the limitations of traditional disinfection products using active ingredients such as bleach or ammonia: these products are only effective while wet; and recontamination of surfaces and skin can occur once the product has dried.
Misconceptions about how long alcohol-based hand sanitisers remain effective for have been brought to light in some studies published online, with some members of the public not realising these products are only effective for around two minutes. Zoono works while wet and continues to work when dry by providing an invisible protective barrier that covalently bonds to a range of surfaces to provide longer-lasting protection against numerous pathogens including bacteria, yeasts, and viruses. A positively charged layer of microscopic pins attracts negatively charged pathogens. This invisible layer of pins causes the cell wall to rupture, resulting in the pathogen breaking up with fatal effect.
The Zoono product range provides a wet-phase and dry-phase killing action. It is important to use Zoono products in conjunction with routine cleaning, applying to a clean, dry surface. In the wet phase, at the point of product application, Zoono works to kill the numerous germs present on the surface. Once dried, the product covalently bonds to the surface, providing longer-lasting protection against potentially harmful germs in its dry phase. The mechanical kill action of Zoono means that the potential mutation of microbes into more resistant strains is not promoted, as can be the case where poisoning or dehydration is used as the mechanism of killing with alcohol or bleach-based products.
Quaternary Ammonium Compounds (QACs) have long been recognised for their long-lasting antimicrobial properties. QACs have been utilised internationally within the healthcare, cosmetic and industrial environments for some time; and many QACs benefit from regulatory approvals globally. QACs present excellent long term antimicrobial activity, while having low levels of toxicity and not leaching over time (Makvandi et al., 2018).
It is well documented that bacteria and viruses can last for long periods of time on hands and surfaces (Hirose et al., 2020). It is also evident that traditional disinfection has a limited disruptive effect, as traditionally used antimicrobial treatments are only active while in their wet phase, allowing recontamination to occur once the surface has dried. Surfaces that look and smell clean can quickly become a source of numerous pathogens, enabling the spread and transmission of disease. A recent study found COVID-19 infection present on a hospital bed was able to spread to 18 other surfaces within 10 hours (Rawlinson, Ciric and Cloutman-Green, 2020). This is where Zoono products come into play. Zoono bridges the gap between routine cleaning processes, modifying the surface to be disruptive to bacteria and viruses between cleaning sessions. Zoono works as part of the greater solution for infection prevention and control (IPC), posing as a new and important tool for the enhancement of IPC in the future.
For our technology to be able to work at its best, it should be applied to clean, dry surfaces; as visible dirt, dust and grease can interfere with Zoono’s ability to bond to the surface. Additionally, while Zoono provides a unique antimicrobial layer of protection to surfaces, routine cleaning procedures should continue to ensure large particles are removed from blocking the antimicrobial layer from being able to work. Zoono is designed to complement the existing cleaning procedure and help reduce the need for harsh chemicals within disinfection. The purpose of the routine cleaning procedure becomes the removal of visible dust and dirt maintaining aesthetic cleanliness, while Zoono remains in place to protect on a molecular level.
Applications
Zoono is colourless and non-leaching and so is suitable for use across a wide range of surfaces in industrial, hospital, transportation, and household settings. Zoono has been trialled in numerous different environments and sectors with great success. When introducing novel technologies it is often the case that seeing is believing, which is why we frequently conduct trials in conjunction with clients or potential clients to prove first-hand the efficacy of Zoono products. Some of the industries that Zoono is used in include:
- Aviation, trains, buses, taxis and cruise ships
- Shopping malls and hotels
- Offices and schools
- Hospitals (including ICUs) and aged care facilities
- Airports
- Warehouses
- Military facilities
- Household use
- All aspects of facilities management
Even high-touch areas in buildings or locations with significant levels of daily footfall have seen significant reduction of the levels of surface contamination present. It has been shown that surface contamination can play a pivotal role in the spread of diseases such as norovirus, Staphylococcus aureus and other germs (Sexton et al., 2018; Wu et al., 2005).
Everyday activities such as flushing the toilet have been shown to disseminate faecal germs onto surfaces within the bathroom (Barker and Jones, 2005). Once on surfaces, these potentially harmful pathogens then have the chance to spread further, via touch, onto new surfaces; and the trend continues. This highlights the importance of long-term protection for both surfaces and hands. Being able to have peace of mind that surfaces do not become contaminated as soon as the product dries is a valuable reassurance throughout multiple industries and sectors.
Surface
It is widely accepted that routine cleaning procedures commonly fail to completely eradicate microorganisms, both in the hospital and wider environments. Therefore, the scope for applying an innovative, durable protective technology to surfaces commonly responsible for cross contamination is enormous.
Application of the product is recommended in one of the following ways:
Fogging
When applying Zoono Z-71 via fogging, it is important to use a fogging machine that can produce a mist at 20 microns or less. It is also recommended that users wear suitable PPE, as specified in the Standard Operating Procedure. Once an area has been fogged, it is important for it to remain undisturbed until the product is fully dry. Benefits of applying the product via fogging include even coverage, application to difficult-to-reach areas and a high product application ratio (1l/80-100m2).
Trigger spray
The recommended method when applying Z-71 with a trigger spray is to saturate a microfibre cloth with Zoono and wipe the desired area(s). This method of application enables an evenly distributed layer of Zoono and provides a quicker drying time. Surfaces treated with Zoono should be left to dry fully prior to touching. It is beneficial to apply the product in this way to high touch point areas for maintenance cleaning.
Use of surface sanitiser should be in conjunction with the normal cleaning routine to ensure maximum efficacy. Zoono should be applied to a clean, dry surface and maintenance cleaning should occur in accordance with the normal schedule, to prevent buildup of large particles such as dirt, dust or grease, which can prevent Zoono’s antimicrobial shield from working by physically blocking it, allowing bacteria and other germs to sit on top of the particle(s).
Hands
Zoono’s hand sanitiser can help protect hands against potentially harmful pathogens for longer. Our hand sanitiser should be applied to clean, dry hands. Once applied and left to dry, Zoono bonds to the surface of the skin to provide an antimicrobial barrier against the many germs hands encounter daily. This product should be used in conjunction with good hand hygiene and is not disrupted by handwashing. Regular washing of the hands remains essential to ensure the product works effectively by removing particles such as dirt, dust or grease from the surface of the hands, allowing the antimicrobial barrier to kill pathogens effectively. Hand sanitiser does not replace handwashing with soap and warm water; instead it acts as an additional layer of protection between handwashing for extended periods of time. This is a vital step forward for infection prevention and control.
Zoono hand sanitiser has been dermatologically tested and rated as ‘Very Good’ with no adverse reactions on a range of skin types. Traditional alcohol-based hand sanitisers are known to cause dermatitis in cases of frequent use, such as the increased frequency applied by hospital staff especially in the unprecedented times of COVID-19. Using an alcohol-free sanitiser in conjunction with good handwashing practices could help combat this growing concern.
Application of Zoono hand sanitiser is as follows:
Apply one pump (3ml) to clean, dry hands and rub together for 30 or more seconds, until dry, and be sure to cover all surfaces of the hands. Apply once daily, or as deemed necessary; and use in conjunction with good hand hygiene.
Cost efficiency
Providing peace of mind and increased levels of protection against potentially harmful germs is where the value of Zoono really comes into its own. Figures from 2016/17 indicate that hospital-acquired infections (HAIs) cost the NHS an estimated £2.7bn in that period (Guest et al., 2020). The exact costs and implications of surface contamination throughout various industries is incredibly difficult to quantify. However, when considering the volume of illnesses that have the possibility to infect people, the knock-on effects stemming from reduced productivity, absence from work, multiple employees becoming ill from the same bug and the subsequent additional stresses inflicted upon the staff who remain at work are just a drop in the ocean. Minimising this risk by using Zoono will help negate the costs associated with surface contamination and spread, and more specifically within the healthcare industries will aid in the reduction of HAIs. In an absenteeism study conducted by a company using Zoono products, it was found that during the winter months Zoono reduced employees’ absence from work by 32.4% on average.
The offset in volume of routine cleaning products used and lower labour costs associated with the use of Zoono Z-71 Surface Sanitiser often reduces the net cost of disinfection practices: independent studies conducted prior to the onset of COVID-19 estimate an average cost saving of approximately £118,000 per month for a facility with an approximate area of 93,000m2. Studies conducted after the onset of COVID-19 found an average cost saving in the region of £9,500 per month per 6,000m2. Cost savings post-COVID-19 included the ability to reduce previously increased frequency of cleaning (labour and materials) and food cost savings by removing individually wrapped items.
Case study examples
ATP refers to the measure of a molecule called Adenosine Triphosphate which is present in all living organisms and is measured in Relative Light Units (RLU). While ATP is a measure of all living matter, it is widely accepted within the food and healthcare industries as a quick, useful measure of environmental contamination.
The above graph shows accumulation of data within different industries which use Zoono. Each data time-point is an accumulation of testing of different areas in different companies and locations into one industry-specific set of data. For example, multiple areas have been tested across numerous public transport locations (airports, trains, buses etc). Each case study selected specifically high-touch areas (for example door handles, handrails, toilet flushes). The pre-Zoono time point refers to testing of the areas prior to the application of Zoono (just having been cleaned as per the normal schedule, with regular products). Post-Zoono (immediate) refers to the testing after Zoono has been applied and left to dry completely (usually one hour post-application); and the subsequent time points refer to days after the initial Zoono application.
The red control line is an accumulation of all the data collected from various industries in which a parallel control group was run alongside the Zoono testing. The control areas continued to be cleaned as per their routine cleaning protocol. Similar areas were used for the control and Zoono-treated areas: for example, the female block of toilets on floor A was used as the control and female block of toilets on floor B was used for the Zoono-treated tests.
We are continually adding to and updating this graph as more in-field testing is completed. Zoono has recently conducted a study in conjunction with an NHS Hospital Trust, which has gained some exciting results and is due to be published as a peer-reviewed article during the coming year.
As can be seen from Fig. 1, there is a significant immediate reduction in surface contamination which either continues to reduce or remains below 100 RLU over the period of testing. From pre-application of Zoono to the end of the test period, an average reduction of 84% in surface contamination can be seen.
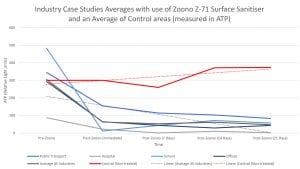
Testing represented on this graph shows results out to 21 days. This is a result of not all testing being conducted out to 30 days, as the testing schedule is outlined at the discretion of the client. Many of our clients choose to have an application cycle of less than 30 days, and so not all testing included within the dataset used for the graph went out to the 30-day mark. However, of the readings which are available at the 30-day mark, there is an average ATP reading of 34 RLU. Significant reduction (88%) from the pre-Zoono treatment average (305 RLU) can be seen, even 30 days after the initial application.
Laboratory results:
Independent laboratory testing of Zoono products has been conducted around the world, with the surface and hand sanitiser products having been tested against the following European (EN) standards:
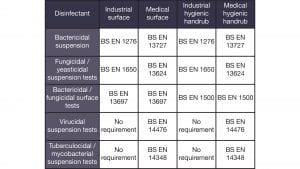
Modified methodologies to extend testing for longevity within laboratory environments have also been conducted. Currently, there is no industry recognised standard for the testing of longevity. Therefore, existing methodologies have been adapted to extend the testing duration. This has been done for up to 24 hours on skin and up to 30 days on surfaces. Test results for our longevity laboratory testing and a selection of our EN testing results can be found on our website (www.zoono.co.uk) under the ‘Our Technology and Efficacy Testing’ section. Zoono Z-71 has been tested for longevity on a range of surfaces including glass, plastics, metal, and tiles. Over 100 tests have been conducted within laboratories globally.
Looking ahead
With COVID-19 causing ever-growing concern, the wider issues of both community – and hospital – acquired infection and antimicrobial resistance continue to persist and worsen, as has been the trend over the last century. Revolutionising the way skin and surfaces are protected with Zoono’s antimicrobial technology can help prevent the spread of potentially harmful germs within the home, workplace, social and healthcare environments. Given health, disinfection and personal protection has been catapulted to the forefront of the media over the last year, there is no better time to invest in advancing both personal safety and the safety of colleagues, clients, patients and visitors.
The time for bold moves is now: utilise this disruptive technology to provide peace of mind and a greater level of protection against the threats that cannot be seen. The increasing threat of existing and novel species of bacteria, viruses and other pathogens give rise to the need to bring innovative solutions to market.
About Zoono
Zoono Group is a global biotechnology company founded in Auckland, New Zealand and listed on the Australian Stock Exchange. Our mission is to improve health and wellbeing through innovative, durable germ protection. Zoono products provide a revolutionary, residual antimicrobial protection technology to help keep you protected for longer. This long-lasting antimicrobial technology has been proven to remain effective for up to 24 hours on skin and up to 30 days on surfaces, based on laboratory test results.
Our products have undergone a wide range of testing, with over 100 tests being conducted in internationally accredited laboratories as well as numerous in-field, industry-specific trials. As our testing is carried out by independent laboratories and our in-field trials are either entirely run by or overseen by clients, these results provide unbiased evidence of Zoono’s abilities. Our in-field testing helps set us apart from other cleaning products by proving real-world efficacy.
As our products are water-based and are alcohol-, bleach- and ammonia-free, Zoono is suitable for use across a wide range of surfaces and environments.
References
1 Barker, J, and Jones, MV. (2005) The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet. Journal of Applied Microbiology. 99 (2), 339-347.
2 Guest, JF, Keating, T, Gould, D, and Wigglesworth, N. (2020) Modelling the annual NHS costs and outcomes attributable to healthcare associated infections in England. BMJ Open. 10, e033367.
3 Hirose, R, Ikegaya, H, Naito, Y, Watanabe, N, Yoshida, T, Bandou, R, Daidoji, T, Itoh, Y, and Nakaya, T. (2020) Survival of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) and influenza virus on human skin: importance of hand hygiene in coronavirus disease 2019 (COVID-19). Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, https://doi.org/10.1093/cid/ciaa1517.
4 Makvandi, P, Jamaledin, R, Jabbari, M, Nikfarjam, N, and Borzacchiello, A. (2018) Antibacterial uaternary ammonium compounds in dental materials: a systematic review. Dental Materials. 34 -(6).
5 Rawlinson, S, Ciric, L, and Cloutman-Green, E. (2020) COVID-19 pandemic – let’s not forget surfaces. The Journal of Hospital Infection. 105 (4), 790-791.
6 Sexton, JD, Wilson, AM, Sassi, HP, and Reynolds, KA. (2018) Tracking and controlling soft surface contamination in health care settings. American Journal of Infection Control. 46 (1), 39-43.
7 Wu, HM, Fornek, M, Schwab, KJ, Chapin, AR, Gibson, K, Schwab, E, Spencer, C, and Henning, K. (2005) A norovirus outbreak at a long-term care facility: the role of the environmental surface contamination. Infection Control and Hospital Epidemiology. 26 (10), 802-810.
Fyn Watch
Sales Director
Jade Pallett
Microbiologist
Zoono UK & Europe
+44 (0)7712 843 118
fyn.watch@zoono.com
jade.pallett@zoono.com
www.zoono.co.uk
This article is from issue 16 of Health Europa. Click here to get your free subscription today.
























