The German Heart Center Munich sets an example for the combination of smart biobanking, biomarker research and data science
High-quality biobanking for tissue and body fluids is considered as a key resource for future research. Many institutions have begun activities to establish new biobank facilities with a special focus on standardised workflows for specimen collection and storage. Unselected collection occurs historically in routine pathology diagnostics, when tissue samples are conserved for later examinations. In contrast, selected study-specific collections are performed in clinical therapeutic trials or for defined biomarker discovery and validation.
However, the collection of biospecimens is only a first step. It is not a value per se to have stored millions of samples in the freezers, although it may have been considered as an attractive business model for some time. More importantly, researchers need access to highly qualified materials to perform meaningful studies. On the one hand, ‘high-quality’ refers to the samples themselves, the collection and storage process, and the documentation and quality control processes. On the other, the clinical context – as well as the quality and extent of the data that is annotated to the single sample – is crucial for use in downstream research.
Biobanking goals and concepts
Biobanking is performed on quite different backgrounds and with different goals. In epidemiologic studies, for example, samples are obtained from normal populations and provide information for the identification of risk groups and early detection of disease. Sample recruitment for clinical studies addresses patients with specific diseases and appropriate control groups to establish the diagnosis of this disease, its characterisation and prognosis.
In cancer, pre-therapeutic samples are highly relevant for the precise stratification of patients to targeted therapy approaches.
Biomarkers used as ‘companion diagnostics’ predict the responsiveness of patients to those treatments, qualifying patients for their application. More sophisticated is the serial collection of samples in defined time intervals for biomarker-based monitoring of the response to therapeutic interventions, as well as for the identification of complications and side effects. Further approaches are the sensitive monitoring of minimal residual disease (MRD) after primary therapy and the early detection of recurrent or progressive disease.
All these applications require smart and thorough biobanking study designs prior to any collection activity to guarantee a successful study outcome and a meaningful answer to the clinical question posed. The following points have to be considered:
- Appropriate groups of cases and controls with sufficient numbers;
- Appropriate time points of sample and data collection;
- Appropriate and standardised pre-analytics;
- Appropriate and validated collection of clinical and outcome data;
- Appropriate and validated methods for biomarker assessment;
- Appropriate established methods as comparison tools to investigate a potential additive clinical value; and
- Appropriate statistics to develop and validate multi-parametric models.
For biobanking aspects, the pre-analytical considerations are most relevant to avoid unknown and unwanted bias on the samples. These would affect even sophisticated and expensive downstream analyses (‘garbage in, garbage out’). The introduction of documentation codes (SPREC), temperature and time stamps – as well as direct sample quality markers – support an efficient quality control management. However, for the many other aspects mentioned above, an interdisciplinary expert team is paramount for a smart study co-ordination.
Value creation by data annotation
While the pure biosamples are quite useless, they gain value by the detailed annotation with clinical data and comprehensive characterisation of the clinical phenotype. Additional information, such as laboratory and imaging test results, and information on the further course of disease, accumulates value over the years. Extensive characterisation by ‘omics’-technologies such as genomics, epigenomics, transcriptomics, proteomics, metabolomics, glycomics, lipidomics, etc. led to a myriad of data points that have to be integrated by sophisticated biostatistical tools. Future developments will include private and social media data that will enable advanced data mining and big data approaches in the context of biobanking. However, it will require an utmost sensibility for privacy and efficient rules for data protection against misuse. This means that smart data science rather than big data will integrate the relevant information whilst respecting individual rights and pave the way for future biobank research.
Complexity of the biobanking enterprise
Several additional aspects in biobanking with regard to ethical, legal and social (ELSI) considerations will certainly gain more importance in the future. Further important fields are donor participation and empowerment, questions regarding informed consent and data protection that develop with the future use of the samples.
Public relations, information campaigns, strategies for sustainable development of biobanking, government of access to samples and data, and networking between biobanks etc. are challenges modern biobanks face. This means that it is not only the management of biospecimen samples, but the management of studies, clinical data annotation, data privacy, additional data integration, financial, legal, ethical, and public affairs that make biobanking a complex, demanding, and highly responsible enterprise.
Currently, there is a gap between the costs and the future return of investment for the society. However, the accelerated development of better diagnostic tools and therapeutic drugs will improve the healthcare system considerably – but then biobanking will become just as normal as other research resources.
Hospital-integrated biobanks
A special situation is given to so-called ‘hospital-integrated biobanks (HIB). These benefit from the already existing logistics of study centres, laboratory services and automation, clinical and laboratory information systems, etc. within a hospital and that are adjusted to the possibilities, needs and clinical questions of a defined hospital. At the German Heart Center Munich at the Technical University Munich, Germany, such an interdisciplinary HIB is being established with the diverse clinical departments and the Institute of Laboratory Medicine.
Planning of the studies, logistics for biospecimen collection and asservation are done by an interdisciplinary team. Physicians then provide patient information and consent, biospecimen collection and clinical data documentation, whilst the handling of samples, aliquoting, storage, administration and co-ordination are all done by the laboratory staff as part of the regular lab logistics. By this means, samples from cohorts of patients with defined cardiac diagnoses and interventions are available that can be used by researchers for basic and applied biomarker research. Importantly, the biobank is part of the Joint Biobank Munich (JBM) and the German Biobank Node (GBN) of the European Network
BBMRI-ERIC, of the Munich Heart Alliance, the German Center for Cardiovascular Research (DZHK) and co-operates with the ESBB in all of which researchers intensively interact with each other.
Biomarker Research Center
One major issue of many biobanks is the rare use of the stored biospecimens. At the German Heart Center Munich, however, the Biomarker Research Center was recently established to promote the use of biofluid specimens for biomarker development and validation and to speed up the scientific process. Although it is a long way from biomarker discovery to the implementation into routine diagnostics, the Biomarker Research Center covers all relevant aspects of this process. In particular, it enables the development and testing of new technologies and biomarkers on:
- Their methodical soundness, including analytical sensitivity and specificity;
- Their robustness towards pre-analytical factors and
- biological variations;
- Their standardisation and harmonisation with similar methods;
- Their utility for application to clinical questions;
- Their validation on independent sample sets;
- Their potentially added value to already existing technologies; and
- Their inclusion into multimarker diagnostic decision panels.
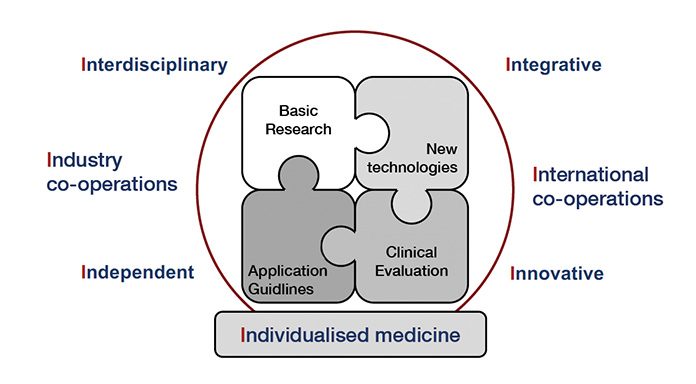
Vision and co-operations
This vision implies, of course, an interdisciplinary approach that integrates other health centres. It is open for industry co-operations to improve the technological basics and works on a national and international level, co-operating with other research groups. It is committed to an independent judgement and applies innovative new technologies on the genetic, epigenetic, miRNA, gene expression, protein, metabolite and cellular level to define the relevant diagnostic tool for future individual decision making
(Fig. 1). In addition, it serves as a biomarker centre for diverse international scientific societies, multicentric clinical trials and standardisation and proficiency testing institutes.
Specific requirements for biomarker assessments of companies or external research groups that are beyond the cardiovascular focus are additionally addressed by the Steinbeis Forschungszentrum BioCoDE (www.stw.de/su/2019) and CEBIO GmbH – Center for Evaluation of Biomarkers (www.cebio-med.com), which has a special expertise in complex study design and highly sophisticated biostatistical evaluation of biomarker datasets. Both work closely with the Biomarker Research Center of the German Heart Center Munich.
The interdisciplinary network of the clinical departments, laboratory, biobanking, basic and biomarker research at the German Heart Center Munich is an example of how sustainable research can be organised in future: Beyond excellent basic research, smart biobanking and data science is certainly key.