
Investment in ultra-low temperature freezers for COVID-19 vaccine distribution and administration is readying the world for a new wave of medicine.
As recently observed in Forbes, despite its overwhelmingly tragic impact on global health and economics, the COVID-19 pandemic will be remembered as the ‘great accelerator’ of technological trends.
With the world’s attention on biopharmaceutical sciences, more funding has been applied to accelerating drug approvals than ever before. Teams of scientists around the world are hard at work advancing and finalising research that has been in development for decades.
With Moderna and Pfizer-BioNTech reinventing the development of present-day vaccines with mRNA solutions, the world is closer than ever to ending this pandemic – but this will not be possible without the support of ultra-low temperature (ULT) storage. This article summarises why there is a newfound need for ultracold storage in healthcare, what we have learned from the COVID-19 pandemic response, and ultimately, why investing in ultracold freezer storage now builds a global foundation for future healthcare needs.
What classifies a temperature as ‘ultracold’?
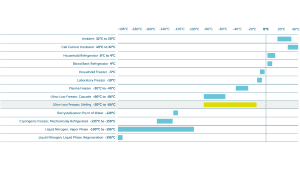
According to the Merriam-Webster dictionary, the term ‘ultracold’ means ‘having a very low temperature; extremely or extraordinarily cold’. To the science community, ultracold storage generally references storage temperatures below -40°C. In more recent media coverage of the vaccine cold chain, the term has been more loosely used to describe any temperature below -20°C. For the sake of this article, we will say ultracold represents -20°C and colder.
Why has ultracold storage become so important?
The potential applications of ultracold storage depend on the capability of laboratory refrigerator and freezer units to reach a range of ‘freezing’ temperatures, the availability of these units, and what the levels of cold they can reach mean for bioprocessing. The colder the environment, the slower molecules and biological proteins move, react or change – this is why foods are frozen to avoid spoilage. Controlling the speed at which these activities happen by controlling the ambient temperature means that important biological reactions, whether within the drug production process or within the patient, only happen when planned; thereby maximising drug effectiveness.
Overcoming cold chain challenges for administering mRNA vaccines is just the beginning
Messenger RNA (mRNA) vaccines deliver instructions to the patients’ cells, teaching them to make a protein which will trigger an immune response in the body and produce antibodies against a selected virus for protection from future exposure. Leading mRNA COVID-19 vaccines use a short snippet of mRNA with the same code as RNA from SARS-CoV-2, the coronavirus that causes COVID-19 (Saey, 20211).
Great advancements in science take time to perfect; and mRNA vaccines, like many other advanced therapies, have proven to be very fragile and unstable at regular temperatures. The speed at which these vaccines have been brought to market has meant that ultracold storage units are required at administration sites to avert the risk of vaccines degrading. This need has been enhanced by the fact that both leading mRNA COVID-19 vaccines, made by Pfizer and Moderna, each require two doses to maximise effectiveness and produce longer-lasting antibodies against future viral attacks.
Recent reports have indicated that the COVID-19 vaccine could potentially become an annual vaccination campaign similar to the ones already in place for influenza, to refresh antibody protection. With the discovery of new disease variants, mRNA developers have gone back to the drawing board to develop new and better vaccine formulae and boosters to cover all strains and release them to world as quickly as possible.
mRNA vaccines were already in place before the onset of COVID-19, but the pandemic has significantly popularised the concept. Another vaccine example which requires ultracold storage is the Merck, Sharp and Dohme Ebola vaccine, Ervebo®, which continues to show great efficacy against Ebola in Guinea. The active ingredient in Ervebo is live Vesicular Stomatitis Virus, with its surface protein replaced with that of the Zaire strain of Ebola, which requires storage temperatures of
-80°C for preservation (Precision Vaccinations, 20212). Ebola is a very deadly disease with transmission rates much higher than that SARS-CoV-2, and must be stopped – but how do remote areas of Africa, where this disease is raging, thermally protect these vaccines without access to dry ice, reliable power supplies or advanced transportation options?
Local storage is paramount; and humanitarian organisations such as UNICEF are partnering with companies like Stirling Ultracold to develop a plan. The vaccines can be shipped to the various storage ‘hubs’ and held in large ultracold freezers until they are transported to local ‘spokes’ for immediate administration with smaller, portable ULT freezer appliances. Stirling Ultracold has named this strategy the ‘hub and spoke’ model.
While this all sounds simple on the surface, ultracold storage is not easy to produce and requires clean, consistent power to maintain. In remote and underdeveloped areas of Africa, South America and Asia this is not always available. To further complicate things, getting vaccines from the hub to the spoke as quickly as possible still presents challenges which logistics companies are trying to rapidly overcome. Even with the fastest delivery service in the world, the need for localised ULT storage will remain.
Why Stirling Ultracold freezers?
Stirling Ultracold freezers, available in three different sizes, deliver the necessary flexibility to adapt to changing ultracold needs. The large upright freezer maximises storage capacity at a centralised hub, taking up less space than a traditional compressor freezer; while the smaller undercounter and portable models provide flexible options for onsite storage at ‘spoke’ sites. Stirling ULT freezers cover the widest range of storage temperatures (-20°C to -86°C); and require reduced input energy because they are powered by a Stirling engine.
Stirling Ultracold freezers provide customers with:
- Infrastructure readiness: Stirling Ultracold freezers offer higher storage densities, while requiring less space, energy, and infrastructure to operate over a wider range of ambient conditions and voltages. These facility infrastructure obstacles, such as floor space constraints, high energy draw, power distribution and limited HVAC capacity, will hamper equitable medical distribution. Additionally, all Stirling freezer models are designed to plug in anywhere – merely by changing power cords, these models can automatically switch across a wide voltage range, including standard outlets from 100 to 240 volts, to operate anywhere in the world without electrical modifications
- Portability for clinical deployment: particularly within the last mile, at onsite clinical administration centers, Stirling’s portable ULT25NEU freezer appliance and SU105UE undercounter unit have proven to be a more reliable alternative than dry ice for COVID-19 vaccine storage. With the flexibility to programme the setpoint temperature anywhere between -20°C and -86°C, these compact units are perfectly positioned for future regenerative medicine and cell and gene therapy programmes where traditional ULT freezers would be too bulky, heavy and require special infrastructure
- Cloud-based temperature monitoring: it is important that temperature logging or monitoring be used to establish a cold chain audit trail. Cloud-based monitoring platforms, such as SenseAnywhere, allow continuous cold chain of custody through multiple stages of the distribution process
New immunotherapies and cancer treatments will require ultracold storage
mRNA, CAR-T therapies, cell and gene therapies, CRISPR, regenerative medicine, monoclonal antibodies, and precision medicine are just a few of the buzzwords popping up in the media lately. Emergency use authorisation for the COVID-19 mRNA vaccines has invigorated the scientific community with additional funding and a newfound confidence that new treatments can be developed, produced and marketed quickly. That means that researchers, engineers and scientists are working faster than ever to safely commercialise advanced therapies to address rare diseases, cure cancers and ultimately extend the health and longevity of the world’s population.
What do all these buzzwords have in common? Their treatments all require some level of ultracold storage to research, to produce, or to preserve long-term for maximum effectiveness. The difficulty is that no two treatments are the same, even within the same drug category. Some may have -20°C storage requirements, while many will fall into the cryogenics category of -135°C or colder. Stability testing to confirm effectiveness and lessen stability conditions takes years to complete and patients waiting for these cures do not have years to wait.
The ultracold chain is becoming a key enabler of next generation medicine
Whether in developmental, translational, manufacturing or patient administration phases of the drug development process, most next-gen treatments will have some degree of temperature-sensitivity, requiring reliable thermal protection to maintain long-term efficacy. Therefore, building a foundation for ultracold chain becomes an essential enabler for critical biopharma developments in the foreseeable future.
References
1 Saey T. (2021, 30 January). Here’s why COVID-19 vaccines like Pfizer’s need to be kept so cold. Retrieved 8 March 2021, from www.sciencenews.org/article/coronavirus-covid-19-why-vaccines-cold-freeze-pfizer-modern.
2 Precision Vaccinations. (2021, 8 March). Ervebo Ebola vaccine. Retrieved 11 March 2021, from www.precisionvaccinations.com/vaccines/ervebo-ebola-vaccine.
This article is from issue 17 of Health Europa. Click here to get your free subscription today.

























