Researchers from the Medtronic Bakken Research Centre in Maastricht, the Netherlands, explain the use of vibrometry in CVD detection
Cardiovascular disease (CVD) encompasses diseases of the heart and of the blood vessels (mainly arteries) connecting organs and tissues. It primarily encompasses diseases of the arteries directing blood to the brain (cerebrovascular disease) and to the heart (the coronary arteries). CVDs and their risk factors are the major contributors to global morbidity and mortality, being responsible for over 17.5 million deaths per year worldwide and representing 31% of all global deaths (WHO, 2012). The early identification of individuals at risk of CVD allows early intervention to delay, halt or reverse the pathological process.
Non-contact laser vibrometer measurement of skin displacement is a technique that can lead to an improved screening and assessment of cardiovascular risk. The technique makes it possible to a) measure aortic and local pulse wave velocity; b) detect vibrations induced by turbulent blood flow in stenosed arteries; and c) pick up cardiac contraction abnormalities via measurements on the chest.1,2,3
A laser Doppler vibrometer (LDV) is an instrument used to make non-contact vibration measurements of a surface. The concept of LDV is quite simple in that an interferometer is used to determine the phase (and thus the optical path length) of laser light reflected from the device under test (DUT). If the DUT is moving, especially if it is vibrating, this phase will perform a harmonic oscillation or a superposition of such oscillations, depending on the particular movement of the DUT.4 The reference and object beams are recombined in a 90-degree optical hybrid and the superposed beams are detected by four photo-detectors (or 2×2 balanced detectors). Polarising beam splitters and a λ/4 plate ensure that the superposed beams are in the same polarisation state (otherwise they would not mix coherently in the photodiodes).
The laser beam from the LDV is directed at the surface of interest (in our case the skin overlying the artery under investigation), and the vibration amplitude and frequency are extracted from the Doppler shift of the reflected laser beam frequency due to the motion of the surface.
This concept offers different areas of implementation:
- Targeting the skin overlying an artery enables the detection of skin vibrations induced by the flow in the artery. A stenosis in the artery will constrict the flow, thereby inducing turbulence and generating vibrations on the surface of the skin where they may be detected;
- Targeting two adjacent points of an artery enables the measurement of the time it takes the pulse to travel between two points of the targeted artery, from which pulse wave velocity (aPWV) can be derived; and
- Targeting the chest allows for the detection of skin vibrations induced by the heart pumping action. Dyssynchrony will change the vibration pattern.
Upon these foundations the objective of CARDIS (Early stage CARdio Vascular Disease Detection with Integrated Silicon Photonics) is to investigate and demonstrate the concept of a mobile, low cost device based on a multi-beam silicon photonics integrated laser vibrometer for the easy targeting of a superficial artery, as well as to validate the concept for the screening of arterial stiffness, the detection of stenosis and heart failure.
Preliminary results
The first achievement was the definition of the user requirements (GPs and MDs in primary care units) that have been set up for the CARDIS prototype based on findings in the literature, as well as through the knowledge of the CARDIS project team.
The user requires a handheld, user friendly point-of-care screening device for a non-invasive detection of arterial stiffness, arterial stenosis and cardiac contraction abnormalities.
The second objective was reached with the system design, subsystems requirements and subsequent integration of subsystems in the CARDIS Demonstrator 1.
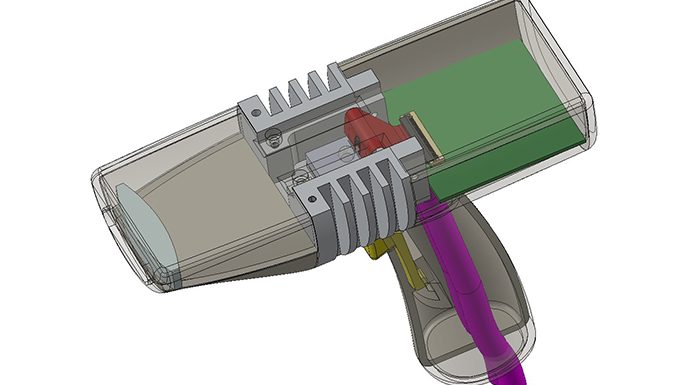
The overall system design for Demonstrator 1 has been provided as block diagrams showing the relationship between subsystems. Demonstrator 1 is designed for non-contact laboratory measurements and will form the basis for the development of Demonstrator 2, which is intended for human trials (Fig. 1).
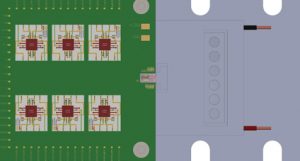
A six-beam, 1,550nm Mach-Zehnder interferometer with integrated detectors has been manufactured on a single silicon photonic integrated chip (PIC) using the iSiPP50G silicon photonics platform at imec. Grating couplers are used for coupling light into and out of the interferometer, which also constitutes beam splitters and phase modulators. A 1,550nm semiconductor laser with beam-shaping optics and a miniature optical isolator is mounted directly onto the PIC for consistent coupling of the laser beam into the interferometer, as well as for miniaturisation and robustness (Fig. 2).
A range of optical systems for beam control and focusing each of the laser beams onto the skin has been evaluated and proven to work.
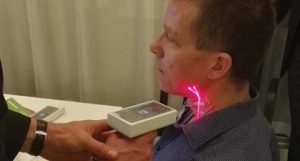
In addition, a human factor engineering (HFE) focus group interview has been conducted within a group of cardiologists, researchers and general practitioners to characterise the best configuration for a reliable usability by the medical professional (Fig. 3).
Reliable simulation
A simple ‘neck phantom’ containing an embedded compliant tube representing the common and external carotid arteries and the measurements of surface displacement has been shown to allow a reliable estimation of PWV. The phantom is currently being implemented with other structures and their effect on signal strength, shape and timing remains is being assessed. The model artery includes the internal/external carotid bifurcation, and a 40% by volume glycerol/water solution will shortly replace the water to more closely mimic blood viscosity.
It was also observed that low amplitude acoustic signals in the frequency range of 100-500Hz are associated with the presence of an axisymmetric stenosis at mean flow rates similar to those seen in the carotid artery. In addition, it was possible to show in vivo that plausible values of carotid artery PWV may be obtained, requiring no direct contact with the subject, and that the shape and timing of displacement signals derived from accelerometers are similar in shape and timing to those from the LDV.
In practice, the initial measurements show the laser vibrometer being capable of measuring the displacement of a moving target with sub μm resolution, beyond the needs of the application.
Current status and next steps
The project has achieved many of its preliminary objectives, with the exception of the PIC chip technology which has been subjected to a five to six month delay as shown in the design of Demonstrator 2; corrective actions for the delay are being implemented to mitigate the impact on the next objectives, i.e. the clinical feasibility currently planned for September 2017.
The technology will be validated in vivo and in vitro by our academic partners (Ghent University, Belgium; Queen Mary University, UK; Maastricht University Hospital, the Netherlands; and Hopital Universitaire Paris Ouest, France) for the three envisioned applications. The concept will be validated in a clinical context as a proof-of-concept study to assess usability and preliminary levels of effectiveness. The proof-of-concept assessment, however, is not a clinical trial.
The measurement of aortic and local PWV
Measurements will be carried out according to the guidelines provided by the Artery Society for the validation of new devices measuring PWV. The study population will consist of 100 subjects equally balanced in terms of gender and covering the age
range of 20 to 80 years to span the range of PWV values. LDV measurements of aPWV will be compared to measurements obtained using the Sphygmocor system, while local carotid and femoral PWV measurements will be validated against ultrasound pulse wave imaging.
The detection of stenosis
Carotid study: Measurements will be done in patients undergoing carotid artery evaluation, either because they are scheduled for carotid stenosis treatment (carotid stenting or endarterectomy) or for cardiovascular risk assessment. We will include 90 patients in total: 30 in the intervention group, 30 having mild stenosis on Doppler (40-70% NASCET), and 30 having no stenosis. For the intervention group, measurements will be performed before and after intervention. In any case, the LDV device will be compared to the ultrasound examination. LDV examination will be performed without knowledge of the results of ultrasound.
Coronary study: Patients undergoing investigation of coronary anatomy either by angiography or CT-scan for the evaluation of chest pain will be included. We will again include 90 patients: 30 having no anatomical stenosis, 30 having mild stenosis on one or more coronary artery (less than 70%), and 30 having severe stenosis (more than 70%) on one or more coronary artery. LDV measuring patterns will be compared pre- and post-treatment.
Detection of cardiac function abnormalities: Measurements will be performed in a group of 50 patients (age 40-70; both sexes) treated for heart failure with a resynchronisation pacemaker. Measurements will be performed with the pacemaker switched off and on, the latter using five different settings and providing five different degrees of treatment. LDV measurements are compared with the simultaneous measurement of finger plethysmography, providing continuous arterial blood pressure and cardiac output information. Moreover, regional cardiac contraction patterns are determined using echocardiography (speckle tracking). Because measurements will be paired (with or without pacemaker) the number to be studied is lower, and so 50 patients is deemed enough for a robust estimation of the effect.
Future perspectives after CARDIS
In CARDIS, we are investigating and developing a medical device by progressing a well-established technology (laser interferometry) towards new markets by using silicon photonics, a newly emerging technology. Medtronic will enter with CARDIS a new market segment, which is primary care diagnostics for CVD. This approach will reinforce the SIOS technology group’s industrial competitiveness and leadership by enabling its current systems to evolve towards small, low-cost, multi-beam systems that can be used in medical photonics (the application of CARDIS), but also in laser-based control systems for manufacturing, safety and security.
The technology developed at imec, Ghent University and Tyndall will be available for other applications and markets such as communications, lighting, manufacturing, medical photonics, and safety and security.
It can be confidently stated that there are no mobile, low-cost devices for reliable, fast and non or minimally-invasive detection of coronary or carotid stenosis, arterial stiffness or cardiac function abnormalities in the primary point-of-care unit. CARDIS aims to find the answer to those aforementioned unmet clinical needs.
References
- M De Melis, U Morbiducci, L Scalise, EP Tomasi, D Delbeke, R Baets, LM Van Bortel, P Segers. A noncontact approach for the evaluation of large artery stiffness: a preliminary study. American Journal of Hypertension, 21 (12), pp. 1,280-1,283 (2008)
2. A Campo, P Segers, and J Dirckx. Laser Doppler vibrometry for in vivo assessment of arterial stiffness. In IEEE Int. Workshop on Medical Measurements and Applications Proceedings (MeMeA), pp. 119-121, Bari (2011)
3. SE Greenwald, HT Banks, MJ Birch, MP Brewin, S Hu, ZR Kenz, C Kruse, D Mehta, J Reeves, S Shaw, JR Whiteman. Acoustic localisation of coronary artery stenosis: Wave propagation in soft tissue mimicking gels. Artery Research. 1 September 2013, vol. 7 iss. 3, p. 124
4. Y Li and R Baets. Homodyne laser Doppler vibrometer on silicon-on insulator with integrated 90 degree optical hybrids. Optics Express, 21 (11), pp. 13,342-13,350 (2013)

























