
2022 could mark a breakout year for XPhyto Therapeutics Corp. as they look at taking their rapid detection of bacterial and infectious disease technology to the next level with biosensors.
Following the recent acquisition of Stuttgart-based 3a-diagnostics GmbH, XPhyto is planning to launch a pipeline of oral biosensors that function as low-cost and easy-to-use oral screening tests. 3a-diagnostics is a world leader in the innovation of point-of-care testing diagnostic tools.
This heralds an important development in that XPhyto is an early entrant in the fast-emerging marketplace for inexpensive, self-testing diagnostic tools that can be used ubiquitously.
The innovation of these next-generation oral diagnostic tools benefits from the financial support of the German federal government. To this point, the Federal Ministry of Education and Research awarded 3a-diagnostics a grant towards the development of enzyme-activated oral biosensors for the rapid detection of influenza A variants that are high-risk pandemic threats. These include H1N1 (swine flu) and H5N1 (avian flu).
The acquisition of 3a also enables XPhyto to integrate 3a’s biosensor technology with its existing proprietary platform of oral, thin-film delivery strips. Originally developed and successfully commercialised by XPhyto’s wholly-owned German subsidiary, Vektor Pharma TF GmbH. This novel drug delivery platform has proven to be well-suited for the precise, metered delivery of pharmaceutical formulations. It now offers considerable promise as a delivery system for oral biosensors.
Company CEO Hugh Rogers says that the anticipated launch of an oral biosensor to detect coronaviruses could be later this year. This breakthrough development would build upon XPhyto’s successful commercialisation in Germany last year of a PCR diagnostics test kit for COVID-19 and its variants.
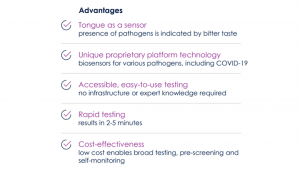
Rogers comments: “We have now achieved the successful identification of the first biosensor candidates to diagnose COVID-19 and its variants. This allows us to expand our portfolio of coronavirus diagnostics, and it complements our recently launched rapid 25-minute PCR test, known as ‘COVID-ID Lab. After optimisation, we can assess the clinical performance of our new candidates and proceed with the commercial development of this novel screening test product.”In terms of their proprietary composition, these oral biosensors are a combination (chemical conjugate) of a pathogen-specific biosensor molecule and an extreme (but safe) bitter compound.
When the biosensor is exposed to specific bacterial and viral enzymes, it triggers biosensor activation and the release of an extremely bitter-tasting compound, alerting the user to the presence of the specific pathogen. In plain language, when these ‘smart strips’ are placed in the mouth, they can quickly indicate the presence of certain infectious diseases, including influenza, COVID-19 and its variants.
This means that they are accurate enough to constitute real-time qualitative screening tools, which can help determine if a person is likely infected and needs to undergo more in-depth testing at a centralised medical triage facility. But they are not meant to be substitutes for full diagnostic tests, which reveal quantitative data, such as viral load or the specific variant.
Other diseases that these biosensors are being developed for include stomatitis, periimplantitis, periodontitis, and Group A strep throat.
Why the biosensor business is booming
Since 2020, there has been a surge in demand for home-based point of care devices due to the COVID-19 pandemic. Even governments around the world are encouraging people to conduct home-based tests on themselves.
Notably, biosensors are ideally suited for self-testing and are destined to earn a large share of this burgeoning marketplace. To this point, the market valuation of biosensors is expected to reach $42 billion by 2027, according to the Delaware-based market intelligence firm Global Market Insights (GMI). This would effectively nearly double the size of the biosensor marketplace within a few short years.
“Increasing numbers of product launches, coupled with rising adoption of novel biosensors will stimulate the global market expansion,” GMI states in a recent research report.
“Early and precise disease diagnosis is essential for the timely prognosis and the survival of the patient. In recent years, the demand for disposable, cost-efficient, and user-friendly devices with a fast response time has extensively increased.”
GMI also says that biosensors are expected to take on an increasingly integral role in containing and curtailing outbreaks of disease.
“Biosensors have emerged in several fields such as diagnosis, patient health monitoring, detection of disease, and human health management, that will further aid in paving robust growth opportunities in the future,” the report continued.
“Their role in fighting coronaviruses cannot be overstated either. COVID-19 has positively impacted the global biosensors market. These advanced biosensors are employed not only for real-time COVID-19 detection but also as a global screening tool to address control, surveillance, and preparedness in the event of future outbreaks.”
3a-diagnostics’ oral screening technology is also designed to instantly detect a variety of other bacterial and viral infectious diseases. 3a’s development pipeline includes biosensor products for COVID-19, influenza A and two deadly variants, stomatitis, periimplantitis, periodontitis, and group A streptococcus.
In terms of proof of concept, 3a-diagnostics has already completed the development last year of its first oral sensor, which is a diagnostic tool for detecting infection in inflamed gums. In Q3 of last year, it was registered under German health regulations for commercial sale in the EU.
Why oral biosensors?
Taking biosensors to the next level
Based in Germany, Vektor Pharma is a European market leader in innovating novel drug delivery systems and has developed new and innovative dosage formulations over the past decade for major pharmaceutical companies. They include the development of generic and hybrid-generic dosage formulations for prescription narcotics.
Vektor has plenty of expertise to leverage off in the development of a diverse pipeline of biosensors for infectious diseases, including coronaviruses.
Essentially, 3a-diagnositics’ enzyme-based biosensors will now be adapted to become ultra-thin dissolvable wafers or film strips that quickly dissolve on or under the tongue, thanks to Vector’s proprietary technology.
Former managing director of Vektor and now running XPhyto’s European operations, Professor Dr Thomas Beckert, sums up the significance of this pivotal development as follows:
“An accurate, low-cost, rapid decentralised screening test that provides immediate pathogen detection would be a powerful and disruptive tool in the fight against pandemics. Such a tool would allow for testing on a scale that is not currently possible.”
A key takeaway here is that the recent acquisition of 3a-diagnostics enables XPhyto to integrate the firm’s disruptive oral biosensor technology with its own proprietary drug delivery platforms, which include thin-film sublingual strips.
As previously mentioned, Vektor’s innovation has been a commercial success to date because it facilitates the optimised uptake of various pharmaceutical drugs, while also ensuring the delivery of extremely precise doses.
Starting this year, XPhyto intends to begin mass-producing its next-generation oral screening tools with a view of selling them at affordable prices to a booming mass consumer market, providing inexpensive, real-time screening solutions for infectious diseases, such as coronaviruses.
Accordingly, the company stands to benefit from an early-market-entrant competitive advantage in a fast-emerging multi-billion-dollar industry.
ABOUT THE AUTHOR
Marc Davis has a deep background in the capital markets spanning 30 years, having mostly worked as an analyst and stock market commentator. He is also a longstanding financial journalist. Over the years, his articles have appeared in dozens of digital publications worldwide. They include USA Today, CBS Money Watch, The Times (UK), Investors’ Business Daily, the Financial Post, Reuters, National Post, Google News, Barron’s, China Daily, Huffington Post, AOL, City A.M. (London), Bloomberg, WallStreetOnline.de (Germany) and the Independent (UK). He has also appeared in business interviews on the BBC, CBC, and SKY TV.


























