
Abbott reflects on the anticipated uptake in rapid, point-of-care technology in cardiovascular and diabetes primary care and proposes steps to smooth the transformation from traditional laboratory pathways to the ‘one-stop-shop’ approach.
The benefits of point-of-care testing (POCT) have been increasingly noted in care pathways around the globe in recent years. Point-of-care testing results are available within minutes enabling patients and their care providers to make management decisions at the time of the patient visit.1-4 This means fewer patients are at risk of being lost to follow up2-3 and practice workflow is improved, leading to operational and economic benefits.5-6 For patients, POCT is more convenient and increases understanding, motivation, and patient satisfaction.1
Point-of-care testing is a particularly valuable tool in disease states requiring screening and continuous monitoring, in addition to diagnoses, such as diabetes mellitus, hypertension, congestive heart failure, and stroke prevention.7
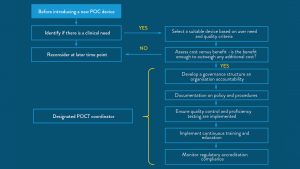
Despite these advantages, widespread adoption of POCT has been slow in some regions. It is critical that barriers to adoption are overcome through training, education, and the sharing of knowledge, including implementation road maps and real-world insights from care providers who have successfully rolled out POCT systems.
Clinical guidance for cardiovascular and diabetes POCT
Several scientific guidelines8,9, including the American College of Cardiology, American Heart Association8 and European Society of Cardiology 10,11, indicate lipid testing for a variety of clinical situations including risk assessment for atherosclerotic cardiovascular disease (ASCVD), selecting patients for statin treatment, diagnosis, and prognosis of ASCVD and patient monitoring.
Meanwhile, regular HbA1c and A1C testing is widely recommended to aid in diabetes management, including by the American Diabetes Association12,13
Poor COVID-19 outcomes are also associated with poorly managed diabetes.14-17 and guidance from the World Health Organization (WHO) has recommended optimising diabetes management.18 Given that POCT has been shown to improve glycaemic control and compliance,19 it may be a valuable tool in this context.
Furthermore, updates to clinical guidelines for cardiovascular disease have increasingly emphasised the lifetime risk of high cholesterol and the importance of prevention strategies to reduce the risk of ASCVD.9,20 They recommend the routine measurement of non-fasting lipid levels to facilitate ASCVD screening and treatment.20,21
Implementing a ‘one-stop-shop’ approach
The ‘one-stop-shop’ approach has been leveraged in both diabetes and cardiovascular settings. 2,22,23
Multidisciplinary management of people with diabetes shows a high level of patient satisfaction where a combined appointment is provided with the local GP, diabetes educator, and podiatrist, plus on-site POCT for HbA1c, albumin-creatinine ratio (ACR), lipids, and glucose.22
POCT is also less costly than the laboratory-led pathway for delivering routine health check screenings for CVD in the primary care setting.2
Cardiovascular disease risk assessment initiatives have found that the ‘one-stop-shop’ approach, where lipids, glucose, and HbA1c are all measured, provides rapid, reliable results, and compares well with laboratory-based methods.23,24
In this context, POCT leads to improved workflow, fewer patients lost to follow-up, optimised glycaemic control, increased patient, and physician satisfaction, and enables early intervention screening.2,3,5,6,19
Overcoming barriers to implementation
Cost perception is often a barrier in introducing a POCT system; however, there is now substantial evidence to show that POCT provides cost savings and economic improvements to workflow and resource utilisation.2 There are also External Quality Assessment (EQA) schemes to provide confidence in the sensitivity and accuracy of POCT.25
Whilst training on the implementation of POCT may also be a barrier to adoption, many devices now available are simple and easy to use.26,1 Road maps and implementation directions are now available to support healthcare professionals in implementation too.25,27
The Afinion™ 2 for diagnosis, monitoring, and management
The Afinion™ 2 Analyzer is a compact, rapid, multi-assay analyser that provides testing at the point-of-care. It enables the implementation of the ‘one-stop-shop’ approach23 in the following ways:
• Multiple analytes in one instrument (including HbA1c, lipid panel, C-reactive protein, and albumin-creatinine ratio);
• Multiple sample types;
• No additional calibration necessary (includes a fixed factory calibration);
• Agreement with laboratory methods; and
• Results within minutes.
The above means that patients can have their tests performed and results given during one visit. Patients can then begin any necessary treatment faster than with traditional laboratory-led testing.23
Learn more about point-of-care testing
Critical to the adoption of POCT pathways, is education and peer-to-peer knowledge sharing, especially where healthcare providers have real-world experience of effective POCT implementation.
myPOCacademy is an educational platform for healthcare professionals, providing free, professionally accredited learning on POCT. Content is certified by the Continuing Professional Development (CPD) service and covers a wide range of learning formats, including:
- Expert webinars (live-streamed and on-demand);
- Expert videos and podcasts;
- Scientific fact sheets;
- Guideline summaries;
- Articles;
- Presentations.

Learners can access content by registering (for free) online via desktop, mobile, or tablet.
Abbott – A Leader in Rapid Point-of-Care Diagnostics.
References
- Kozel, Burnham-Marusich. J Clin Microbiol. 2017;55(8):2313-2320.
- El Osta, et al. BMJ Open. 2017;7:e015494.
- Patzer, et al. J Diabetes Sci Technol. 2018;12(3):687-694.
- Abel. Expert Rev Mol Diagn. 2015;15(7):853-5.
- Lewandrowski, et al. Clinica Chimica Acta. 2017;473:71-74.
- Crocker, et al. J Diabetes Sci Technol. 2020;15(3):561-567.
- Goble, et al. J Pharm Pract. 2017;30(2):229-237.
- Arnett, et al. Circulation 2019;140:e596-e646.
- Grundy, et al. Circulation. 2019;139:e1082-e1143.
- Mach, et al. Eur Heart J. 2019;41(1):111-188.
- Consentino, et al. Eur Heart J 2019;41(2):255-323.
- Riddle, et al. Diabetes Care. 2020;43(1).
- Johnson, et al. Diabetes Care. 2019;42(Suppl. 1):S1-S194.
- Barron, et al. Lancet Diabetes Endocrinol. 2020;8(10):813-822.
- Bornstein, et al. Lancet Diabetes Endocrinol. 2020;8:546-50.
- Holman, et al. Lancet Diabetes Endocrinol. 2020;8:823-33.
- ADA, 2022. Available at: https://www.youtube.com/watch?v=QbqSznLDDCE
- WHO, 2020. Available at: www.who.int/publications/i/item/clinical-management-of-covid-19
- Schnell, et al. J Diabetes Sci Technol. 2017;11(3):611-617.
- Visseren, et al. Euro Heart J. 2021;42:3227-3337.
- Mora, et al. JAMA Intern Med. 2019;179(7):898-905.
- Shephard, et al. Rural Remote Health. 2005;5(3):371.
- myPOCacademy, 2021. Available at: www.mypocacademy.com/events/the-one-stop-shop-point-of-care-lipids-impact-on-cardiovascular-disease-screening-and-management
- Jain, et al. Ann Clin Biochem. 2017;54(3):331-341.
- Lenters-Westra, et al. J Diabetes Sci Technol. 2019;13(6):1154-1157.
- St John, et al. Clin Biochem Rev. 2014;35(3):155-67.
- NHS, 2017. Available at: https://www.dbth.nhs.uk/document/corprisk8/
© 2022 Abbott. All rights reserved. All trademarks referenced are trademarks of either the Abbott group of companies or their respective owners. Any photos displayed are for illustrative purposes only. Medical editorial support is provided by Healthware International. COL-12150 05/22
This article is from issue 22 of Health Europa Quarterly. Click here to get your free subscription today.








