Speaking with Health Europa Dr Daniela Marino explains personalised skin graft, how CUTISS came to be, along with sharing the many challenges in the world of skin defects.
Severe and extensive skin defects present tremendous difficulties to medical professionals, as the standard of care involves the harvesting of healthy tissue in order to obtain a skin graft to heal the wound (autografting). When a large percentage of the skin is damaged it is very challenging to harvest sufficient autografts – an issue known as donor site shortage. Little can be done to quickly and optimally treat patients with large third-degree burns; but this could be about to change with Cutiss’ personalised skin graft solution.
Cutiss has developed a potential solution known as denovoskin, a personalised skin graft which can be developed in large quantities with only a small biopsy required from the patient. denovoskin is currently undergoing phase 2 clinical trials to assess its efficacy, and it can also be accessed by surgeons across Europe who are in need for therapy to treat compassionate use patients. Health Europa Quarterly spoke to Dr Daniela Marino, CEO and co-founder of CUTISS about the progress of the trial, the challenges it has presented so far, and the potential future applications of the revolutionary denovoskin product.
What inspired the creation of CUTISS and the denovoSkin solution?
The denovoskin product was invented at the university of Zurich more than 10 years ago. The surgeon who came up with the idea used to operate on many children in Zurich and he was disheartened that there was no innovation or developments which could improve the way they were treated so he decided, together with scientists, to establish the Tissue Biology Research Unit (TBRU). I joined the TBRU in 2009 as a post-doctoral researcher working on blood and lymphatic vessels, and I was asked to try and obtain funding through EU grants so that we could advance with the denovoSkin project.
Soon after, we received €6m to continue to help bring denovoskin from bench to clinic. This was a big challenge; a big project and the EU would actually push us to think about the future of the product all the way up to its commercialisation. Thus, I started to become familiar with topics like business plans, IP, study protocols, project management, pricing, and I enjoyed it. When we treated the first patients and saw the promising results, I had the vision: I would have done all in my power to ensure that people around the world could access our technology.
Overnight I decided I wanted to be the CEO of a startup and be the person to bring this to market. I had no experience whatsoever, but I wanted a chance because I am a good communicator and I thought I may have the right skills to succeed in the position, so I took the leap and since then Cutiss has gone far.
denovoSkin™ is a personalised bioengineering dermo epidermal skin graft, but what makes this treatment different than the other treatments and solutions in the industry?
Essentially, at the moment no matter how rich you are and no matter what part of the world you live in, if you burn yourself in the third degree and over a large surface area there is only one treatment which will help you to recover from your burn and this is thin, meshed autografting.
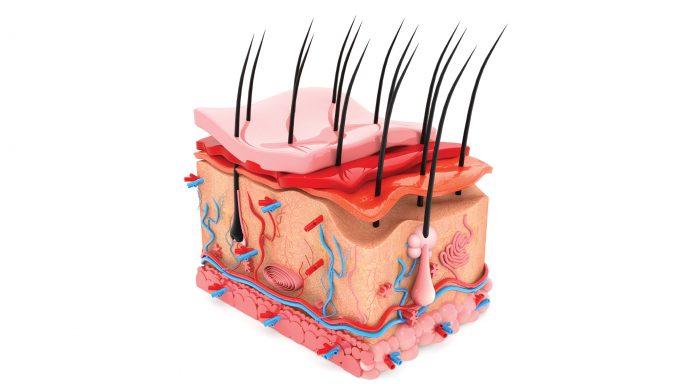
If you burn your left arm, the surgeon uses the right arm to take the skin from to graft onto the wound. Unfortunately, this means that the surgeon must create a new wound where the skin was previously healthy. In addition, the autograft is much thinner than the wound (it contains the epidermis but only remnants of the dermis).
Therefore, the body creates a scar underneath and induces contraction which results in the scarring outcome. Scars are unfunctional tissue, so they are disfiguring, debilitating and they can be painful and impair mobility and growth. Scars require follow up surgeries and intense homecare. If you have a little wound you can live with that, but if half of your body is gone then your life as you knew it is gone. From the moment you burn yourself you are a burn victim, and from the very next day until the day you die you are a burn survivor which really is not an easy way to live your life.
DenovoSkin can also help burn survivors by replacing their scars and improving the scar quality. Indeed, denovoSkin can also be used to treat patients who need skin grafting for other reasons (e.g. tumours, resection, plastic surgery etc.)
There is no other product like denovoSkin on the market. Commercial products (e.g. acellular templates) can help as fillers before autografting occurs in the case of very deep defects. Commercial epidermal grafts or suspensions (in combination with autografting) can help in case of severe burns affecting an extremely large part of the body (e.g. 80%) but the final outcome is still not satisfactory.
denovoskin has a chance of being the first product which actually reaches the market offering a real alternative to autografting, a personalised bioengineered graft that can be manufactured in large quantities from a small piece of skin from the patient. Because we use bioengineering, we can create a graft which can fill the wound correctly since it is thick and composed of both dermis and epidermis.
Let’s talk about the clinical trials; how will the study examine and compare the safety and efficacy of the denovoSkin transplant product? Will this differ between the burn clinical trials and the reconstruction clinical trials?
We have already completed a phase one trial – so a safety trial- on children and we have transplanted denovoskin onto children who were burned and required transplantation, but also onto children who were burned in the past and wanted to remove the scar. Thus, we already have data on acute cases (burns) and on elective cases (reconstructive). We are currently running the phase two trials one for burns and one for elective cases, and with both protocols we are comparing on the same patient denovoskin versus the standard of care in a randomised body location. Results will be available in the next 1-2 years.
What kind of challenges are you expecting from the clinical trials? How would you best overcome them?
The biggest problem with burns is that they are completely unpredictable, so we need to include many centres, and this comes at a cost. Right now, we have four centres in two countries which can recruit patients, but we are attempting to involve Italy, Belgium and Germany this year to try and speed up recruitment.
The reconstructive trial is different in that sense because we can actively recruit patients. Patients, professionals and investors now have the chance to support our efforts to achieve our goal: giving access to our technology to people in need.
What does the future hold for CUTISS and the denovoSkin treatment?
We want to be different. We really have a huge challenge in trying to bring a personalised tissue therapy to the market. I am increasing the complexity even further by wanting to bio-engineer skin which is our largest organ. To be successful, we need to be able to produce reliably, in a robust and cost-effective way and in large quantities. We are working on this full speed and we are already preparing for the scale up.
We have a fully equipped R&D lab working on characterising the product and we have a team working on automating the manufacturing element. We need to be able to produce using machines because otherwise we will not be able to scale up, decentralise and reach out to the patients. In addition to the automation project, funded by EU and CH grants, we are also building our new manufacturing facility and building very strong manufacturing expertise. Our journey is extremely challenging at times but ultimately, we have a huge opportunity to produce something which will change the lives of many patients and that is why we will keep doing our best to succeed.
Once the trials are completed do you have any ideas for other potential applications? Could it be modified accordingly for different conditions?
If the results of the current phase two trials are good, then we could go straight to conditional market validation to treat burns. Once we are able to collect and extrapolate more data then we can start looking at the reconstructive applications which means we could potentially treat anybody with any indication. The other thing we can look at is chronic wounds such as ulcers, this is also a potential indication for denovoskin. That would be a completely different approach compared to the work we are doing now, it’s like a different planet. They are small wounds which don’t heal and are usually found in elderly, obese or diabetic patients and are triggered by their pathology, so the onset is a completely different story.
We are aware of the fact that it is an enormous market due to the aging population, but it is also very crowded. There are a huge amount of potential treatments so although it is definitely a space we could enter, but at the moment it is not our focus as we need to ensure Cutiss is well established and take it one step at a time. Any investment at this stage would really help us to lay a strong foundation from which we can grow and develop – the future possibilities could be endless.
Dr Daniela Marino
CEO
CUTISS AG
+41 76 230 8046
daniela.marino@cutiss.swiss
www.cutiss.swiss
Please note, this article will appear in issue 10 of Health Europa Quarterly, which will be available to read in July 2019.