Real-world evidence gathering is central to devising a patient-centred medical cannabis strategy.
In the world of clinical research, real-world data (RWD) and real-world evidence (RWE) are gaining in popularity; as concepts, they represent an important and accelerating shift in attitudes for the ways in which new treatments are developed and evaluated. They support the move towards patient-centric and value-based care – but what does ‘real-world’ actually mean? What can this data be used for? Why should we care and what comes next?
In the last couple of years alone, a number of well-funded startups have emerged inspired by the success of real-world evidence pioneer, Flatiron Health – which was sold to Roche for $1.9bn in 2018.
Flatiron integrated, cleaned, structured and analysed real-world data pulled from the clinical notes of primarily community-based oncologists. This is a particularly challenging task as real doctors’ notes can include a lot of shorthand, abbreviations and esoteric terms. Once a clean dataset had been prepared, Flatiron could make that data available to cancer drug developers to help them uncover new indications for their treatments with the goal of ultimately obtaining regulatory approval to market their drugs to an expanded patient population.
Flatiron had a couple of standout successes. One of the first was helping Pfizer expand the indication of its breast cancer drug Ibrance to include men. Ibrance was given its first approval in 2015 for the treatment of breast cancer in women only.
In a response to online newsletter The Cancer Letter, the US Food and Drug Administration (FDA) explained that, in its consideration of the approval, ‘the RWE endpoints used were real world tumour response and safety data. Real world tumour response was taken from the electronic health record as part of routine clinical care and information about each response event was retrospectively collected. Therefore, this response included several factors, such as physical exam, symptom improvement, and pathology reports, which were used to supplement descriptions of radiology findings in the overall clinicians’ assessment of response.’
Two features of this decision mark this as a seminal moment in drug development. Firstly, the FDA based its decision on clinical evidence which that was generated outside the ‘gold standard’ evidence generating apparatus – the double blind randomised clinical trial (RCT) – indicating a willingness to alternative sources of evidence to inform major approval decisions. The approval also implies that there was sufficient ‘off-label’ drug use occurring amongst these community oncologists to support a meaningful conclusion. After all, enough oncologists must have been trying the treatment in men – essentially off-label – to build statistical power. This really gets to the heart of why there is such a growing interest in making use of RWE: that ‘real’ patients frequently do not look like trial participants.
‘Real-world’ (non-trial participant) patients are complex; and real-world health services are not consistent either. We expect our regulators to approve treatments which are safe and effective for the typical patient populations they are intended to treat. Not just the carefully curated cohorts of most clinical trials.
Can RWD and RWE help approximate the real-world variances of patients and health services?
Global regulators like the FDA and the European Medicines Agency (EMA) are starting to look more critically at how clinical evidence is prepared and delivered to support medical product claims or, as they are more formally called, ‘market authorisations’. RCTs are under increasing scrutiny. There is a growing number of cases where key results from RCTs have not been replicated in real-world populations. As a result, there is an increasing interest in real-world evidence to support medical claims.
The great hope is that, by using more real-world data, we can improve the generalisability of research: this is why major international regulators are preparing guidance for industry on how to use RWE in market authorisation decisions; sponsors, drug developers and contract research organisations are investing heavily in RWE programmes; and startups are sprouting up to support these efforts.
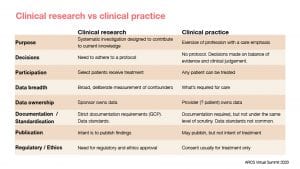
Today, when we talk of RWD and RWE, we mean the kind of data and evidence that are generated by the increasing digitisation of healthcare and supported by advancements in cloud-based computing power, data integration technologies and analysis techniques. Most of the time, we are talking about using data which is spun off as a byproduct of modern health services where care, not evidence, remains the primary emphasis (see table).
Emyria’s model
At Emyria, we are on a quest to build a clinical model that pushes the care and data quality frontier – that is, a model which will provide great care while also improving our capacity to learn from every patient. We are developing technologies and clinical workflows designed to maximise the quality and utility of the clinical evidence we collect from every patient. It’s something more than real-world evidence – a better description might be ‘authentic world evidence’ (AWE).
Key features:
- Intended for patients with unmet medical needs, where access to trial or experimental treatments is desired
- Patients invited to consent to research participation upfront
- Patient health goals captured upfront to measure treatment success
- Patients invited to control their data
- Broad set of validated clinical and patient-reported outcome measures used as endpoints – helps address the inability of database research to measure the impact of a broader set of clinical factors, which is a recognised weakness
- Doctors are trained in good clinical practice principles to improve data capture consistency and quality
- Clinical trial-grade data management principles, practices and technologies adopted
- Clinical encounters are slow and deliberate to ensure sufficient clinical evaluation is performed
- Remote monitoring technology used to track patients outside of the clinic
- Emyria clinicians use insights from Emyria’s own patients to guide decision making –we try to learn from every patient
- Emyria clinicians involved in in-house technology development efforts to improve engagement and likelihood of successful adoption
One of the first applications for Emyria’s model is in the study of cannabinoid-based medical products (CBMPs) which were suddenly made available in Australia as an unregistered, prescription-only medicine in 2016. In order to develop the product-specific clinical evidence required for registration, Emyria set up Emerald Clinics.
Emyria’s model in practice: the Emerald Clinics case study
Evaluating cannabinoid-based medicines using authentic world evidence
Depending on the source, cannabinoid-based medicines are either the next miracle cure-all or an over-hyped substance with no obvious clinical benefit. This tension exists because CBMPs have become available to patients and doctors unaccompanied by the typical clinical evidence packages and prescribing guidelines we expect from new drugs. Consequently, major knowledge and competency gaps exist across the entire health ecosystem, involving producers, patients, clinicians, payers and regulators. This leaves CBMPs vulnerable to equal parts exaggerated exuberance and stubborn scepticism.
This unprecedented situation has also created a set of commercial and clinical imperatives for new, knowledge-generating care models that can provide safe patient access to CBMPs while also producing robust clinical evidence to help guide their use.
CBMPs are purported to have broad clinical applications, from helping manage chronic pain; to improving sleep; to reducing seizure frequency in childhood epilepsy, to name a few. However, very few licensed CBMP producers are investing in the substantial time and resources required to conduct large-scale RCTs and conclusively prove the safety and efficacy of their products. Couple this with an increasing variety of products and formulations, as well as some ‘regulation-naive’ promotional practices, and you have clinicians and regulators who are understandably cautious about making any endorsement as to what to prescribe, for whom and for how long.
In Australia, this has meant that CBMPs are carefully titrated by the Therapeutic Goods Administration’s (TGA) Special Access Scheme (SAS): a compromise which allows for limited patient access (after a lot of case-by-case argument and significant out-of-pocket expense), but which prevents widespread prescription. However, despite the bottlenecks, patient demand for broader access to CBMPs is growing, particularly amongst a not insignificant number of patients experiencing profound benefits taking CBMPs after years of ineffective ‘standard of care’ treatments (consider the presentation of a chronic pain patient requiring ever larger doses of opiates). As a result, we are faced with an intriguing challenge: how can we provide access to patients in need, but at the same time generate the kind of quality clinical evidence that can correctly inform clinicians, regulators and payers of the true clinical and pharmacoeconomic benefits to CBMPs over the long term?
The Emerald Clinic model is explicitly designed to tackle this by using technology and novel clinical routines so that the data collection and management disciplines common to clinical trials can operate within the typical, busy care setting. It is Emerald’s goal to capture the experience of every patient so that their data can contribute to improving the global knowledgebase for where CBMPs sit in the therapeutic set.
Implications and hopes for the future
Emyria’s belief is that new models of care can safely reveal legitimate medical indications for novel treatments, like CBMPs, without having to commit the vast human, capital and time resources required to complete RCTs. The increasing global acceptance, interest and investment into RWE provides an opening under which we can build new models of care which are explicitly designed to generate robust and ethically sourced clinical evidence, with the co-operation of patients.
We believe the technology exists to do this well, but that the biggest barriers to adopting these models will be organisational. New business models and partnerships will be required to create the right behavioural and economic incentives. We will need a way to support clinicians as they invest their time upfront in order to avoid missteps down the road. High quality data capture, analysis and sharing, if done right, could help reveal the true benefits of all health interventions sooner.
Perhaps more interestingly, the model could also demonstrate a new way of practising medicine: one which is less transactional and more knowledge-creating. One which more deeply invites the patient into the decision making and treatment evaluation process, more often. In this way, we can start to think of evidence creation not so much as the purposed of contrived trials (RCTs), or as the fortuitous byproduct of an increasingly digitised but still highly transactional healthcare system (RWE), but as part of the central purpose of care delivery.
The FDA’s definitions of real-world data and evidence
Real-world data (RWD) refers to the data relating to patient health status and/or the delivery of healthcare, which is routinely collected from a variety of sources. RWD can come from a number of sources, for example:
- Electronic health records (EHRs)
- Claims and billing activities
- Product and disease registries
- Patient-generated data including in home-use settings
Data gathered from other sources that can inform on health status, such as mobile devices
Real-world evidence (RWE) is the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of RWD. RWE can be generated by different study designs or analyses, including but not limited to randomised trials, large simple trials, pragmatic trials, and observational studies (prospective and/or retrospective).
Dr Michael Winlo, MBBS (Hons), MBA
Managing Director
Emyria Limited
www.emyria.com
This article is from issue 15 of Health Europa. Click here to get your free subscription today.