CorVent Medical is delivering a simple solution for resource-limited institutions to increase the ventilatory capacity to save lives.
The COVID-19 pandemic was a rough wakeup call for many in the healthcare industry. It accelerated a growing concern around our collective strategic preparedness – we found that preparation is not a reactive response, but one that requires time to forecast and build for the future, whatever that future may bring. This planning, however, comes at a cost that is difficult to project from a ‘what if’ scenario. The questions we are left with are these: what did we learn, and where do we go from here?
When capacity in hospitals reached critical mass at the peak of the pandemic, it became apparent that the high cost of ownership for life-sustaining ventilators, along with the recurring maintenance and service requirements, made readiness preparation and deployment of these devices more difficult than it should have been. Add in resource-constrained factors such as the burn rate of high-flow oxygen consumption, lack of trained users, or limited ICU space, and the ability to treat patients throughout the pandemic was dramatically compromised.
CorVent Medical is a US-based company founded in early 2020 in direct response to the COVID-19 pandemic, with the goal of developing a cost efficient ventilator for critical care use. While originally developed in response to heightened surge events, CorVent’s RESPOND 19 ventilator measures up with any ICU ventilator on the market. Designed with an easy out-of-the-box setup that requires no calibration, a simple user interface, and no requirement for high-flow oxygen, the RESPOND 19 ventilator delivers on a singular focus: increasing the capacity to save lives.
How a pandemic can impact your ICU
Capacity is just one of many factors which contributed to the impact that the pandemic had on our hospital systems, and in particular, our critical care units. In a pulse survey conducted by the United States Department of Health and Human Services (HHS), other challenges such as severe shortages of testing supplies, widespread shortages of PPE, difficulty maintaining adequately trained support staff, and anticipated and realised shortages of ventilators all had a damaging effect on the emergency response for hospitals.
When the spotlight shifted to the shortage of ventilators in hospitals, it opened a conversation around the distribution, operation, and efficacy of full-feature ventilators for critical care use. For smaller public and private hospitals where stockpiled ventilators were not readily available for use, alternative options were utilised by shorthanded staff, such as adapting anaesthesia machines and emergency transport ventilators for use in ICUs. In addition, many intensive care specialists, frontline nurses and physicians trained to operate both standard critical care ventilators and their alternative options became infected with COVID-19, resulting in inadequate staffing to support patient surge. Lastly, many hospitals found that while full-feature ventilators may have been stocked, that did not mean that they were fully operational – without keeping up with the proper routine maintenance and cadence of service, these life-saving devices were inoperable and failing at a remarkable rate.
Surge events, such as the early months of the COVID-19 outbreak, impact all levels of the healthcare system. They also invite us to think critically about managing our preparedness for future events. On one hand, surge events come and go, but the need to remain prepared is paramount to manage the initial peak stages of a future situation. On the other hand, finding solutions to further simplify the cycle of care mitigates the potential panic often associated with unexpected critical events. For the critical care ventilator space, RESPOND 19 offers a solution to both.
Introducing the RESPOND 19
The RESPOND 19 ventilator is a lightweight, electrically powered mechanical ventilator that provides mandatory, assisted and spontaneous breath types for ventilator-dependent adult patients. It is intended for use in a hospital, or similar resource-constrained clinical environments, by qualified, trained personnel under the direction of a licensed physician.

The greatest advantages of RESPOND 19 lie in its ease of use and reliability.
- For the clinician, peer feedback and review demonstrated that training for operational use of the RESPOND 19 ventilator could be taught in less than an hour
- For the frontline user, all user-adjusted settings and alarm limits can be easily adjusted and monitored through an LCD screen simply by following the quick reference guide on top of the ventilator
- For the hospital administrator, the modular and ultra-durable design allows for optimal storage conditions for up to five years without the need for routine maintenance or service costs
The reliability of the RESPOND 19 is a function of its simplicity, utilising less than 120 components to deliver a cost effective, ICU-ready ventilator. Each internal component has been carefully selected and chosen for rated lifespans of greater than five years. Extra manufacturing steps have been added to ensure reliability, such as adding gold-plated contacts for all electrical components. With hundreds of thousands of hours of non-stop validated reliability testing, the RESPOND 19 ventilator has not presented a single mechanical or electrical failure. These results demonstrate reliability that is 10 to 20 times greater than other ventilators in the market today.
Ensuring a high level of reliability without maintenance or service contracts begins with the elimination of the need to replace internal components typically embedded within critical care ventilators, such as a battery or internal flow sensor. The REPSOND 19 does not contain internal components that require routine service or maintenance.
The RESPOND 19 also requires a very low amount of energy, utilising only about 25W of power to operate. This is opposed to the typical 750W to 1000W requirement of other ICU ventilators, which constitutes the need for an internal battery to operate. This low power consumption provides the RESPOND 19 with the distinct advantage of being able to use an outside uninterruptible power supply (UPS) for at least three hours of continuous ventilation during a power outage or intra-hospital transfer.
To support a patient through the cycle of illness and assist them in returning to full health, the flow sensor of the RESPOND 19 is located externally to the ventilator. By attaching directly to the patient circuit wye, it offers the most accurate and reliable flow triggering when ventilating a patient. Designing these components outside of the ventilator precludes the need for maintenance and calibration, along with the advantage of being easily replaced as needed by any hospital staff. Innovations like these provide confidence in the design and function of the RESPOND 19 ventilator and allow hospital staff to focus their efforts on ensuring patient recovery during a critical care event.
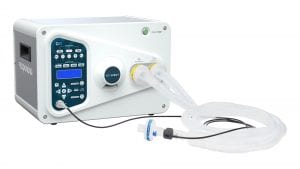
Supporting a greater capacity
During times of emergency, ensuring critical care capacity in hospitals is the key to saving lives. The RESPOND 19 is ideal for transforming any hospital floor into an ICU unit. In any hospital room that contains a bed, a wall outlet, and a low flow oxygen source, the RESPOND 19 can be added effortlessly to convert that room into a useable critical care space. In cases where capacity in the ICU is capped, the option to convert unused rooms into ICU beds can serve as a great benefit for the hospital.
A method to ensure faster deployment of ventilators in a future emergency, particularly those in more rural or undersized hospital regions, is to prepare with your own stockpile. The size of the RESPOND 19 ventilator is designed specifically for easy and optimal storing conditions. Weighing less than 6kg and modular in shape, units can be stored on top of one another until needed – and ultimately, when the time comes for a lifesaving response, these ventilators can be both deployed and ventilating a patient in under 15 minutes.
To accommodate the range of patients needing treatment, the RESPOND 19 offers both invasive (IV) and non-invasive (NIV) ventilation modes. In NIV mode, the flow sensor located at the patient wye provides the added benefit of supplying accurate flow-based triggering in the presence of leaks due to its robust triggering algorithm. Because it is located next to the patient, its accuracy for leak compensation can be continually measured without calibration.
Responding to critical care events quickly
As many of us experienced, the panic buying of ventilators in reaction to a global pandemic resulted in acute shortages, unfulfilled orders, and ultimately lost lives across many institutions. Ultimately, the takeaway from the COVID-19 experience was that we need to be more prepared to tackle events before they arise and to have already sourced reliable, low-cost solutions to meet these impending needs. There is now an intense pressure to identify solutions at a reduced cost, but also with improved quality and reliability.
Expanding the ability to operate complex medical devices such as ventilators can further improve the response time for a patient in need. Traditionally, specialised week-long training was necessary to train physicians and nurses on new ventilator technology and platforms, but in critical times where staff is limited and time is of the essence, lifesaving knowledge needs to be disseminated much more quickly and simply to be utilised effectively.
Lastly, technology must be optimised to focus on the critical ventilator modes that can save, sustain, and wean patients for decreased ventilation time during their cycle of care. Faster flow triggering, accurate oxygen delivery, and improved reliability are all critical to the execution of these modes, but to do so with a simple solution that can be managed and handled with ease will further improve patient outcomes and move resources more quickly to the next patient that needs critical ventilatory support.
CorVent Medical is committed to bridging the gaps in each of these areas to improve both the hospital experience and positive patient outcomes. With the low-cost, low-power, no-maintenance solution found in the RESPOND 19, we address the most pervasive concerns that stemmed from COVID-19. Our disruptive and innovative technology may be the ideal solution to these unmet institutional needs, and we encourage you to demo it for yourselves. Reshaping the critical care space is of utmost importance to engage in now – not only to increase capacity during future surge events, but also to ensure better patient results in the ICU when resources are again truly limited.
This article is from issue 18 of Health Europa. Click here to get your free subscription today.























