The Vaccine Adjuvant Research team at Statens Serum Institut has the mission of developing novel vaccine adjuvants.
Vaccines are by far the most successful medical intervention in history, and only access to clean water and sanitation has led to a bigger improvement in life expectancy. The first generation of vaccines consisted of whole-inactivated, attenuated or disrupted (split) viruses or bacteria preventing diseases such as small pox, tuberculosis (TB; BCG vaccine), measles, polio (OPV/IPV vaccines) and influenza. Besides containing the vaccine antigens, all these vaccine technologies also contain immunostimulatory components that activate the innate immune system to increase and modulate the adaptive immune response to the antigens. Another strategy has been to isolate, purify and detoxify toxins directly from the pathogens – a method applied in the diphtheria and tetanus vaccines.
Common for many of these vaccines is that they demand production of the pathogens themselves, although often in an attenuated form. The obvious production-related disadvantages of this approach have been circumvented by implementation of recombinant production methods using benign expression systems such as E. coli to produce the protein-based vaccine antigens with high yields.
Vaccine adjuvants
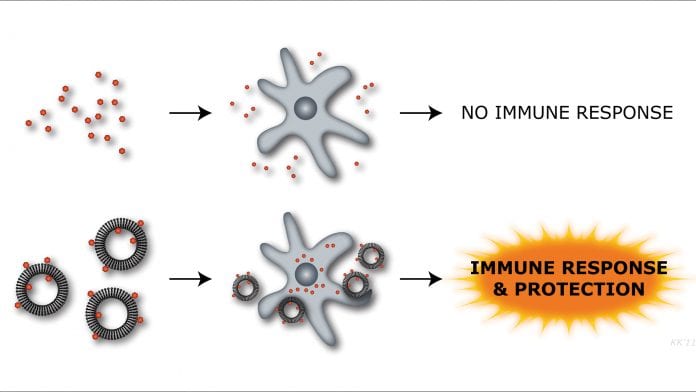
These highly purified and recombinantly produced vaccines are not able to sufficiently activate and potentiate the immune response by themselves. Adjuvants are therefore added to supply the ‘danger’ signal and trigger innate immune activation and vaccine potentiation.
The diphtheria and tetanus vaccines contain the adjuvant aluminium hydroxide, which potentiates an antigen-specific antibody response needed to protect against these infections. This adjuvant has been the most widely used since the 1930s when it was first introduced. In fact, since then only a small number of adjuvants based on other mineral salts, squalene emulsions and virosomes have been approved for use in humans. A common feature of these adjuvants is that they significantly increase the humoral antibody response to vaccine antigens but are not able to mount the type of cell-mediated immunity (CMI; see box) that is important for protection against many of the most challenging infectious diseases today, such as tuberculosis, chlamydia and pandemic influenza.
Modern vaccine adjuvants, such as the AS01 adjuvant used in the newly registered Shingrix® vaccine (GSK) against varicella-zoster virus-induced shingles, consist of a delivery system, typically based on emulsions or liposomes carrying special molecules that are recognised as foreign by the immune system.
These immunostimulators are typically synthetic analogues of so-called ‘pathogen-associated molecular patterns’, or PAMPs, and can modulate the immune cells to react in a specific way. They can, for example, shift the induced antibodies towards special functions or induce a certain type of T-cell with special ability to kill infected cells (so-called ‘killer T-cells’ or ‘cytotoxic T lymphocytes’ (CTLs)) or modulate homing of immune cells to specific tissues, such as the intestines or lungs.
The majority of immunostimulators in clinical development activate receptors of the so-called ‘toll-like receptors’ (TLRs) or ‘C-type lectin receptor (CLR) family’ and will have a major impact on the efficacy of novel vaccines, especially in those cases where a strong CMI response is required for protection.
Statens Serum Institut
The Danish Statens Serum Institut is a state-owned enterprise under the auspices of the minister of health, and located close to the city centre of Copenhagen, Denmark. Statens Serum Institut is responsible for Denmark’s preparedness against infectious diseases, involving disease surveillance through international collaboration, and consultation with the Danish healthcare system and authorities in the event of epidemics that demand urgent action.
Vaccine research at Statens Serum Institut
Vaccine research at the Statens Serum Institut goes back to the institute’s founding in 1902, when it was set up to produce antisera for diphtheria. Research soon expanded to other epidemic diseases. Vaccine research at Statens Serum Institut is focused on diseases that represent a major threat to global health. Today the main effort is devoted to vaccines against tuberculosis, chlamydia, HIV and pandemic influenza.
The vaccine research programme is an integral part of SSI’s core mission with respect to vaccine preparedness and supply. The major part of the vaccine research conducted at Statens Serum Institut is placed under the Center for Vaccine Research and covers the entirety of vaccine development, spanning from hypothesis generation and basic research to clinical evaluation of vaccines in humans. The centre focuses on immunological responses to infection and both basic and translational vaccine research, involving detailed antigen discovery programmes aimed at identifying the proteins expressed by the target pathogens and recognised by the immune system.
Presently, the two major strategic vaccine programmes are directed towards developing novel vaccines against tuberculosis and chlamydia.
Tuberculosis is one of the leading infectious killers in the world today and caused approximately 1.6 million deaths in 2017, including 230,000 children. The Bacillus Calmette-Guérin vaccine effectively protects children and is given to babies as close to the time of birth as possible in countries where tuberculosis is common. The efficacy of BCG, however, wanes over years, and it typically has little protective effect when the children enter the teenage years. There is therefore an urgent need for vaccines that can improve protection against TB, especially in adolescents and adults.
Chlamydia trachomatis is one of the most prevalent sexually transmitted infections and there were 1.7 million reported cases alone in the United States in 2017.
Unfortunately, this infection is seemingly heavily underdiagnosed, with many countries not reporting disease rates, making it difficult to get an exact overview of the burden. It can cause permanent damage to women’s reproductive systems, potentially leading to fatal ectopic pregnancy or infertility.
Some serotypes of Chlamydia trachomatis furthermore cause trachoma, infection under the eyelids, which can eventually lead to blindness. This disease is a major health issue in Africa, Asia, and Central and South America, where it causes blindness of 1.2 million people and reduced vision in an additional one million people.
Both pathogens infect cells and the vaccines against them thus demand adjuvants that induce a strong CMI response.
Vaccine adjuvant research
For the last decade, the Statens Serum Institut has developed new vaccine adjuvants alongside being involved in the development of vaccines against both tuberculosis and chlamydia.
The aim of our research is to tailor the adjuvant to induce the exact immune response needed to control the pathogen in question. This is possible through the construction of adjuvants based on liposomes that incorporate immunostimulators. The immunostimulators are derived from naturally occurring PAMP molecules from micro-organisms that trigger different parts of the immune system. The properties of these liposomes can be modified, and they can be produced in different sizes and with different molecules incorporated depending on the immune response needed for a specific vaccine. The tailor-made liposomes are combined with the vaccine antigen in the final vaccine. The liposome will ensure that the vaccine antigen is presented to the right cells of the immune system and that the desired immune response is being generated.
In our pursuit of the ideal adjuvant for a vaccine antigen we systematically modify the composition of the vaccine delivery system and/or the immunostimulators. We modify the composition of the delivery particles in order to change physical characteristics of the vaccine delivery system, e.g. in terms of size, fluidity or charge. Such modifications can be used to ensure optimal antigen adsorption, vaccine depot, in vivo uptake and presentation etc. The liposome fluidity can, for example, have profound influence on the distribution of the vaccine components and the level of CMI and antibody responses.
In our search for the optimal immunomodulator we have found that several mycobacterial lipids have strong immunomodulatory effects which can be exploited for vaccine development. We have been involved in the discovery of α, α´ trehalose 6,6´ dibehenate (TDB) as an effective immunomodulator for induction of cell-mediated immune responses and the dissection of the signalling pathways involved.
The immunomodulator discovery programme has also identified the mycobacterial monomycoloyl glycerol (MMG) as an effective activator of human dendritic cells and as an immunomodulator, giving rise to a prominent Th1 response in animal models. In this discovery programme it has also been important for us to understand the innate mechanisms activated by these novel immunomodulators. Finally, the route of vaccination is also under evaluation in the group and has been shown to have a major impact on the immune response induced. We are therefore working with different immunisation strategies, including delivery to the upper airways, to improve protective immune responses at mucosal surfaces.
The CAF adjuvants from SSI
Our adjuvants are all based on positively charged liposomes and are thus called cationic adjuvant formulations (CAFs). The first adjuvant formulation developed in our lab consists of liposomes formed of dimethyldioctadecylammonium (DDA) stabilised with the synthetic mycobacterial immunomodulator TDB, which is inserted into the lipid bilayers.
DDA acts as a delivery vehicle serving to promote uptake and presentation of the vaccine antigen in the relevant subset of antigen-presenting cells (APCs), while TDB acts as an immunomodulatory, activating APCs to induce combined Th1 and Th17 CMI responses. Together the two components, DDA and TDB, act in synergy to generate highly potent T-cell and antibody responses shown to be effective in vaccines against a range of different diseases, e.g. in animal models of melanoma, influenza, chlamydia, tuberculosis, group A streptococcus and malaria.
CAF01 has been tested in five Phase I clinical trials to evaluate safety, tolerability and immunogenicity of different doses of CAF01 administered in combination with various protein and peptide-based vaccines (clinical trial nos. NCT00922363, NCT01009762, NCT01141205, NCT02787109). These studies showed that CAF01 is both safe and effective at inducing vaccine-specific T-cells, which play an important role in protection against, for example, tuberculosis, chlamydia, malaria and pandemic influenza.
Our second-generation adjuvant, CAF09, which consists of DDA, MMG and polyIC, was shown to be very effective at inducing antigen-specific cytotoxic T-cells against protein and peptide-based antigens. This adjuvant is thus a potential candidate for vaccines against, for example, HIV, cancer, pandemic influenza etc. It is now going through clinical evaluations in humans in a therapeutic vaccine against prostate cancer (NCT03412786) and a neo-antigen-based cancer vaccine against various cancer types (a so-called ‘basket trial’, NCT03715985).
Building on the principles of CAF01 and CAF09, we can further modulate the formulation when designing tailor-made adjuvants for specific disease targets through changing of the cationic liposomes or by incorporating different immunostimulator combinations into them.
Currently we are combining mycobacterial nonTLR ligands with conventional TLR ligands in the different delivery vehicles. Our aim is to induce highly diverse and complex immune responses, and by adjusting the different parameters we have demonstrated that we can influence the antibody isotype, the duration of vaccine depot, and the CTL/Th1/Th17 balance.
Research disciplines
Vaccine delivery and formulation is a multidisciplinary project spanning both applied and basic research at the highest international level. This includes:
- Biochemical and physicochemical characterisations of adjuvant systems
- Studies of the distribution and fate of vaccine formulations in vivo
- Basic immunological characterisations of adjuvant function in vivo and in vitro
- Characterisations of vaccine-induced immune responses in humans and animal models
- Protective immune responses in different challenge models.
Currently, we have protein- and peptide-based vaccine projects within the fields of TB, influenza, chlamydia, HIV and group A streptococcus, as well as therapeutic melanoma and human papilloma virus vaccines under evaluation. In addition, we have extensive experience within preclinical development of adjuvanted subunit vaccines and are involved in supporting first-in-man trials of novel adjuvants.
Collaborations
The research is carried out in collaboration with research groups from Danish and international universities, biotech companies and governmental institutions.
Important previous and current
collaborations include:
ADITEC
This high impact project ran from 2011-2017 to develop new vaccination strategies. The scope of the project, funded through the Seventh Framework Programme (FP7) of the European Commission, was to accelerate the development of novel and powerful immunisation technologies for the next generation of human vaccines. ADITEC has made significant advances in the development of novel immunisation technologies, adjuvants, vectors and delivery systems, formulations and vaccination methods optimised for different age groups.
TRANSVAC2
This collaborative infrastructure project was funded by the European Commission’s Horizon 2020 programme. It is a joint effort between leading European groups working in the field of vaccine development and is designed to accelerate vaccine development by enhancing European vaccine research and training, and to increase the sustainability of European Commission vaccine projects by implementing a permanent research infrastructure for early vaccine development.
BIOVACSAFE
This project funded by the Innovative Medicines Initiative (IMI) ran from 2012-2018 with the goal of developing cutting-edge tools to speed up and improve the testing and monitoring of vaccine safety, both before and after release to the market. By bringing together three of Europe’s leading vaccine development and manufacturing companies, as well as top experts from academic institutions and SMEs, the project has generated an enormous amount of results that can accelerate the development of a new generation of safer, more effective vaccines.
TBVAC2020
This Horizon 2020 research project aims to innovate and diversify the current TB vaccine pipeline. The project builds on the highly successful and long-standing collaborations in subsequent European Commission FP5-, FP6- and FP7-funded TB vaccine and biomarker projects and brings together scientists and developers from 40 research partners to collaborate on developing novel TB vaccines.
NeoPepVac
This project, funded by Innovation Fund Denmark and involving four partners, aims to generate personalised immune therapy vaccines based on peptide neoantigens in combination with the CAF09b adjuvant designed to provide optimal immunotherapy through CTL induction.
The project will complete a Phase l trial with neoepitope-based immunotherapy in cancer patients and provide proof of concept for the overall strategy, safety and clinical feasibility. Based on in-depth analysis of immune reactivity in vaccinated patients and identification of neoepitopes using syngenic mouse models, we will improve neoepitope prediction algorithms for future treatments.
UNISEC
This European Commission FP7-funded consortium included 11 partners from academia, public health institutes and the vaccine industry.
It combined expertise in influenza virus and vaccine production, vaccine formulation, vaccine administration, preclinical animal models, immunological read-outs, clinical trial organisation and execution, data management and data analysis to compare different novel influenza vaccine concepts in order to identify, develop and clinically test the most promising leads for a universal influenza vaccine.
ENOVA
This science and technology ‘Network on Vaccine Adjuvants’ was funded by COST through the EU programme Horizon 2020. ENOVA brings together European experts and stakeholders working in different areas of adjuvant and vaccine R&D, including both prophylactic and therapeutic applications as well as human and veterinary vaccines.
The ultimate goals of the network are to facilitate communication and the exchange of information among its membership, to ensure that new discoveries are widely disseminated so that their potential can be of optimal benefit, to promote the best use of existing adjuvant technologies, and to encourage and support the development of novel adjuvants.
The SSI proprietary adjuvants mentioned in this article can be acquired for preclinical as well as clinical exploitation by contacting Statens Serum Institut. See contact information below.
Facts on adjuvants
Adjuvant: From the Latin adjuva¯re; to help. A substance that enhances the immune response stimulated by an antigen when injected with the antigen (Collins English Dictionary).
About the author
Dennis Christensen is senior scientist and leader of the vaccine adjuvant research at Statens Serum Institut. He is furthermore visiting professor at the University of Strathclyde, Institute of Pharmacy & Biomedical Science, in Glasgow, UK.
He has a PhD in pharmaceutical sciences and has for the last 15 years been working with pharmaceutical and immunological aspects of vaccine adjuvants and delivery systems, including targeted delivery of immunostimulators and antigens.
Highlighted recent publications
- Pedersen GK et al. Immunocorrelates of CAF family adjuvants. Semin Immunol 2018;39:4-13
- Schmidt ST et al. Induction of Cytotoxic T-Lymphocyte Responses Upon Subcutaneous Administration of a Subunit Vaccine Adjuvanted With an Emulsion Containing the Toll-Like Receptor 3 Ligand Poly(I:C). Front Immunol 2018;9:898
- Vono M et al. Overcoming the Neonatal Limitations of Inducing Germinal Centers through Liposome-Based Adjuvants Including C-Type Lectin Agonists Trehalose Dibehenate or Curdlan. Front Immunol 2018; 9:381
- Christensen D et al. Seasonal Influenza Split Vaccines Confer Partial Cross-Protection against Heterologous Influenza Virus in Ferrets When Combined with the CAF01 Adjuvant. Front Immunol 2018; 8:1928
- Christensen D et al. Vaccine-induced Th17 cells are established as resident memory cells in the lung and promote local IgA responses. Mucosal Immunol 2017;10(1):260-270
- Schmidt ST et al. The administration route is decisive for the ability of the vaccine adjuvant CAF09 to induce antigen-specific CD8+ T-cell responses: The immunological consequences of the biodistribution profile. J Control Rel 2016; 239:107-117
Dennis Christensen, PhD Pharm
Head of Vaccine Adjuvant Research
Center for Vaccine Research
Statens Serum Institut
+45 3268 3804
den@ssi.dk
https://en.ssi.dk/research
Please note, this article will appear in issue 8 of Health Europa Quarterly, which is available to read now.

























