
When medication is consumed it must reach the intended site of action, but what is the degree and rate at which it is absorbed by the human body? NNE spotlights a vital concept in pharmacology: bioavailability.
Speaking to Christian Carlsen, Business Director of NNE and Morten Allesoe, Principal Consultant at NNE, Medical Cannabis Network gains an insight into the importance of achieving cannabinoid bioavailability, along with understanding ways new therapeutic medications can be developed and monitored to provide effective medication to the patient.

NNE is an independent engineering and consulting company, supporting the entire pharmaceutical manufacturing life cycle from setting direction, realising benefits of strategic investments and optimising manufacturing and business processes. NNE advises medical cannabis companies on their journey from the initial concept, through developing a business case, establishing a facility; and developing and delivering quality medical cannabis to patients.

For a while now, the cannabis industry has been focusing on investments and global expansion; and less on the human benefits of medical cannabis. Allesoe, pharmacist and Principal Consultant, along with Carlsen, explore the effectiveness of focusing on bioavailability to not only demonstrate how this could provide improved medication to patients but also benefit businesses in the long term.
The challenges of achieving bioavailability
“Generally speaking, bioavailability is the degree to which a drug or other substance becomes available to the target destination [in a living system] after administration,” explains Allesoe. “In this particular case, we are dealing with cannabinoids and specifically THC and CBD.” Having worked for big pharmaceutical companies with huge exposure to R&D for more than 10 years and now as a consultant, Allesoe described the importance of increased focus on the part of the medical cannabis industry on the optimisation of pharmaceutical products in relation to the human body. Currently, a high fraction of the total dose in cannabis medicines consumed orally is wasted due to its poor absorption of THC and CBD by the body. Due to the way these cannabinoids are absorbed into the body, further technology and medicines need to be made available to allow patients to reduce their dosages significantly and enable a more robust release.
Alongside bioavailability, it is equally important to understand cannabinoid pharmacokinetics, which refers to the absorption after diverse routes of administration and from different drug formulations, distribution of the active components throughout the body, metabolism by the liver and extra-hepatic tissues, and elimination in the faeces, urine, sweat, oral fluid, and hair.1 Understanding the pharmacokinetic profile of a drug is essential to understanding the onset, magnitude and duration of its effects, maximising its therapeutic benefits and minimising negative side effects.
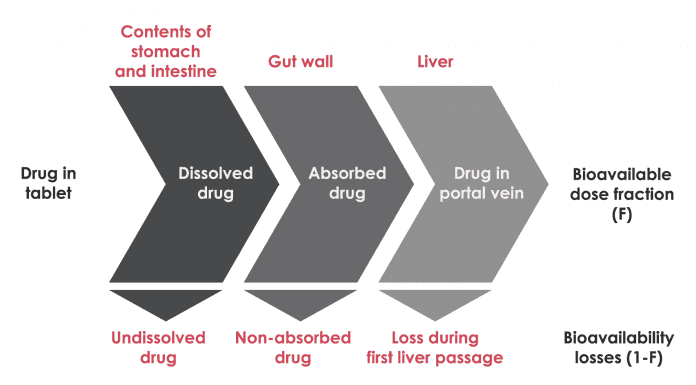
The challenge posed by CBD and THC is their poor water solubility, meaning that a large fraction of the dose may be lost in the gut and not absorbed. Even if cannabinoid extract is mixed with an oil (in a capsule) it may precipitate out of solution from the oil in the gut, thus lowering the absorption.
If the formulation technology, such as lipid carriers or nanoemulsions, are effectively optimised to have high bioavailability, then upon consuming a drug the contents would have a higher absorption rate; thereby maximising the proportion of the drug which reaches the required destination and achieves systemic circulation. Allesoe adds: “One of the aims of achieving high bioavailability is that you have to make sure that a high percentage of the dose reaches the blood circulation from which it may travel to the sites of action.”
If the drug molecules are not correctly formulated in the dosage form, or alternatively redesigned to withstand first-pass metabolism in the liver, this not only reduces the effectiveness of consuming the medication, but also increases the number of dosages a patient must take to treat whatever ailment they are experiencing – or worse yet, the patient may not get an effect at all.
Allesoe explains that the optimal formulation technology addresses the challenges of bioavailability reduction at all stages:
- Improving dissolution in the gut;
- Increasing the absorption across the gut wall; and
- Reducing the extent of first-pass metabolism.
As detailed in Fig. 1, the liver is an organ which the molecules pass through via the portal vein when a drug is administered orally. Following its passage through the liver, the drug enters the systemic circulation from which it enters the site(s) of action for therapeutic efficacy. Many foreign molecules entering the liver undergo some extent of enzymatic conversion as a means to eliminate the molecule; and the fraction lost here also contributes to a reduction in overall bioavailability.
Allesoe adds that injection directly into the bloodstream is far more effective: “the best example of a drug that has 100% bioavailability is when you inject this directly into the bloodstream, where you actually have 100% bioavailability. Of course, this is regarded as the most inconvenient route of administration by patient and is not a realistic formulation strategy for cannabis products. But nonetheless it must reach the blood.”
Bioavailability and formulation strategies
Carlsen says: “Currently, the bioavailability aspect of drug development in the cannabis industry has been less of a priority. It has been much more business focused and less clinically focused.” As a result of this, by pushing out some products which work well for patients alongside others which have not undergone rigorous clinical testing to meet high market demand, some cannabis products currently commercially available are suspected of having a low bioavailability – and more importantly, with a poor level of efficacy.
Multiple factors may account for the low oral bioavailability, including variable absorption, degradation of drug in the stomach; and significant first pass metabolism to active 11-OH-THC and inactive metabolites in the liver.1 However, if drug development focused on creating medications with better absorption rates – deploying methods such as formulation technology and molecular modification to improve absorption of cannabinoids, improving absorption or focusing on the potential of nanoemulsions – there may be a better offering of increasing the bioavailability of cannabis drugs.
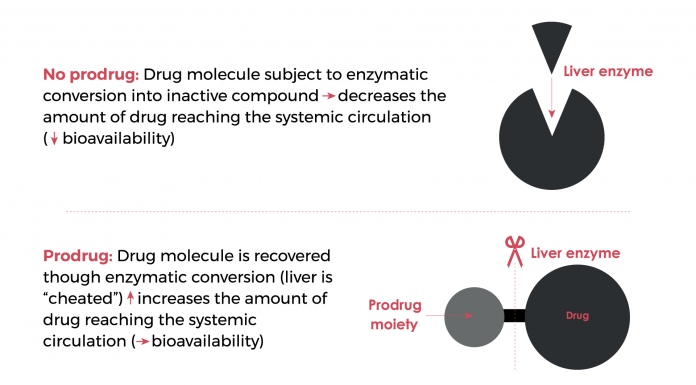
Allesoe says: “When you consider the life cycle management [of a drug], you look at gaining the most continuous value to maintain patient interest in your product; of course, only when added benefits to the patient can be demonstrated. For the sake of improved clinical efficacy, the concept of molecular modification into prodrugs may be relevant to the cannabis industry in the future.”
Prodrugs are derivatives of therapeutic agents created to improve the pharmacokinetics profile of a drug. Within a prodrug, the pharmacological activity of the drug is masked and is recovered within the human body upon bioconversion of the prodrug in the liver, a process that is typically mediated by enzymes.2
The prodrug strategy can improve bioavailability after absorption turning the otherwise negative effects of first-pass metabolism into an advantage (Fig. 2). However, molecular modification of a drug into a prodrug is just one strategy. It is expensive; therefore, some first-pass metabolism is acceptable. Although there is little research regarding the extent of first pass metabolism of THC/CBD, Allesoe adds that it is something that should be investigated further to find the right product formulation strategy. That being said, molecular modification through chemical synthesis is a science on its own and does require extensive research.
The value of focusing on bioavailability
Carlsen explains: “We are trying to make our clients aware of this concept, as they need to understand its importance both in relations to the therapeutic effects of their products but also their business. By this I mean if you have a high bioavailability you need a smaller portion of the drug, thereby reducing dosage for the patient and reducing cost of production; in contrast when you have a very low bioavailability, patients or consumers would need a higher dosage of the drug to gain the similar effect.”
Allesoe adds: “We are essentially trying to help businesses in the long term to understand the importance of bioavailability; and the ultimate benefits it will bring to not only businesses but also patients and consumers.”
Did you know that NNE are Partners with Medical Cannabis Network? Discover their Partner Profile here: NNE Partner Profile
References
- Huestis, M. A. (2007) ‘Human Cannabinoid Pharmacokinetics’ Chemical and Biodiversity [online] 4 (8), 1770–1804. available from <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2689518/> doi:10.1002/cbdv.200790152.
- Walther, R., Rautio, J., and Zelekin, A. N. (2017) ‘Prodrugs in medicinal chemistry and enzyme prodrug therapies’ Advanced Drug Delivery Reviews, Elsevier [online] 118, 65-77. Available from <https://www.sciencedirect.com/science/article/abs/pii/S0169409X17300972>
Christian Carlsen
Business Director
NNE
+45 4444 7777
czca@nne.com
Tweet @NNEglobal
https://www.nne.com/
This article will appear in the second issue of Medical Cannabis Network which will be available to read in April 2020.


















