Following the sharp uptake in hand sanitiser products during the COVID-19 pandemic, Professor Nigel Silman, Visiting Chair in Infectious Disease at the University of the West of England, explores the efficacy of this, now commonplace, infection control measure.
The emergence of the SARS-CoV-2 virus, the causative agent of COVID-19, prompted the introduction of a number of public health measures to try and control the spread of the virus. One of these was the widespread use of hand sanitisers, and this has now become a common practice among individuals and continues to be an entry requirement for a wide variety of public places such as hospitals, GP surgeries, shops, and theatres.
Preventing the transmission of COVID-19
In the very early stages of the pandemic, the main routes of virus transmission and the efficacy of topical sanitisers and disinfectant chemicals were unknown. Subsequent scientific research indicated that, unlike the influenza virus (where approximately 50% of transmission is via fomites and then hand to face), SARS-CoV-2 is almost entirely transmitted by infectious aerosol particles exhaled by infected persons, but good hand hygiene remains an important control measure in the transmission of all infectious diseases.
The other key finding was that, in keeping with other well-characterised Coronaviruses, SARS-CoV-2 was not readily inactivated by alcohol (ethanol and propan-2-ol) based disinfectants unless extended contact times of up to 10 minutes were employed.1 The caveat here is that contact times of one minute resulted in approximately a 3-log reduction (logarithmic reduction is the universal way of describing disinfection efficacy, and thus a 3-log reduction would reduce the number of viable organisms by a factor of 1,000) in viable Coronaviruses when concentrations of ethanol between 62 – 71% were used. All of these tests were performed according to statutory surface disinfection protocols where metal or other non-porous materials are coated in virus particles and then immersed in the disinfectant solution for the required time and the viable virus particle number remaining is determined.
Although this is a standard test method, it does not accurately represent the way in which hand sanitisers are used. The other point to note is that many of these studies evaluated chemical disinfectant formulations which are never intended for use on or in humans, only on inanimate surfaces, so again these data need to be carefully examined before recommendations are made and conclusions are drawn.
Indeed, the US Environmental Protection Agency (EPA) publishes a list1 (list N) of compounds for use in disinfecting inanimate surfaces and 48% of these use quaternary ammonium compounds alone and a further 10% use quaternary ammonium compounds in combination with at least one other active ingredient. Within this list, only 6.4% of compounds contain alcohol as an active ingredient; this list contains EPA-approved products either directly tested against SARS-CoV-2 or against surrogates where results may be extrapolated to SARS-CoV-2.
The key to effective disinfection
Disinfection is the term used to describe a process that reduces the number of pathogenic microbes (knock-down) present on a surface (or it may, in some instances completely remove them) or in a solution, whereas sterilisation is the complete removal of pathogenic microbes. Sanitisation is essentially the same as disinfection and describes the reduction or complete removal of pathogenic microorganisms.
For surface-acting disinfection agents to work effectively, there are two critical considerations. The first of these is the concentration of the active compound and the second is the contact time. For effective bacterial and viral knock-down by alcohol-based sanitisers, a concentration of between 62 and 71% of the alcohol is required. In order of effectiveness, propan-1-ol is the most effective alcohol disinfectant, followed by propan-2-ol and then ethanol and most alcohol hand sanitisers use ethanol1,2 as propan-2-ol is more typically used in surface disinfectants or wipes to sterilise the skin surface prior to injection.
In order to exert its disinfectant properties, alcohols require water to be present in the formulation as they act by denaturing proteins in the bacteria or viruses, so somewhat counter-intuitively, higher concentrations of alcohols are less effective.1 Despite this, there are a number of products available that use up to 90% or 95% alcohol in their formulation. Thus, we have already seen that the active ingredient concentration is critical to optimal performance measured in terms of microbial reduction or knock-down.
The second key factor is the contact time that any disinfectant is required to be in contact with the material to be disinfected or sanitised. Here is where the use of ethanol in many hand sanitisers may be questioned. A recent review1 of the effectiveness of a range of hand sanitisers with varying alcohol concentrations as well as those which are alcohol-free indicated that a minimum contact time of 30 seconds was required to inactivate a range of enveloped viruses, including SARS-CoV-2 and reduce the number of viable viruses by between 3 and 5-logs but that a contact time of one minute resulted in greater efficacy. Herein lies one of the potential problems with alcohol hand sanitisers, in that these extended contact times for effective reduction of pathogenic microorganisms on the skin may never be reached due to the inherent volatility of the active ingredient, alcohol.
Formulating alcohol hand sanitisers with varying concentrations of glycerol results in increased contact times but with the negative effect that it leaves the skin surface sticky, which is obviously a contra-indication for skin cleanliness.7 This same study also suggested that the widespread and frequent use of alcohol hand sanitisers could result in oral, dermal and/or pulmonary absorption and subsequent systemic toxicity from the alcohol.7
One of the other important factors to consider when using skin sanitisers is the duration of action. Alcohol hand sanitisers have recently been shown to have a very short duration of action and, therefore, protection against re-contamination.1 Moreover, alcohol hand sanitisers can cause a number of unwanted side effects such as skin dehydration, contact dermatitis and skin cracking. In fact, a number of studies looking at the effectiveness of alcohol-based hand sanitisers on the removal of Noroviruses indicate exactly the opposite and that use of alcohol-based sanitisers actually increases the risk of Norovirus outbreaks.8,9
The prevalence of alcohol hand sanitisers
So, the question remains, why have alcohol-based hand sanitisers continued to be so widely used? The answer lies in the evidence base surrounding the introduction of hand sanitisers into routine clinical practice. We must not forget that our skin is the largest human organ and given its direct contact with the environment, is frequently exposed to environmental microorganisms in addition to its own natural bacterial microflora.1
Microorganisms live both on the skin surface and within the living epidermal layer; recent evidence also now indicates that this microflora comprises a small number of viral species,11 so it is important to understand that irrespective of the amount and degree of hand-washing and use of hand sanitisers, most of these commensal species will not be removed. The entire reason for hand hygiene is the removal of pathogenic microorganisms capable of causing disease and being transmitted (particularly in a healthcare setting) to more vulnerable persons.
It has long been recognised that the hands of healthcare workers may provide a reservoir for the circulation and transmission of drug-resistant bacteria and other pathogenic microorganisms in the hospital environment.11 Conventional hand washing with soap and water is an effective means of reducing the microbial burden of pathogens such as Staphylococcus aureus which has been found to colonise between 10 and 78% of healthcare workers’ hands with up to 1 x 10 bacteria present.10
In situations where access to soap and water is not available, bodies such as the US Centers for Disease Control (CDC) have recommended the use of hand sanitisers containing either 80% ethanol or 75% propan-2-ol. Evaluation of the World Health Organisation (WHO) recommended formulations alongside a large number of commercially available alcohol hand sanitisers indicated that whilst the WHO formulations resulted in complete bacterial inactivation within one minute of exposure (only bacterial strains were tested, not viruses) most of the commercially-available, over the counter preparations required at least five minutes exposure, which as we have seen earlier does not occur with alcohol-based products due to the rapid evaporation of the alcohol.13 Interestingly, this same study also indicated that the presence of thickening agents such as glycerol further impacted the efficacy of the bacterial kill and the authors hypothesised that this was due to the slower release of the active ingredient.
What type of hand sanitisers should I use?
The question we have not yet addressed is, what are the alternatives to alcohol hand sanitisers? The answer has been alluded to earlier in this article, in that the EPA List N hand sanitisers list contains products which largely (93.6%) do not use alcohol as the active ingredient.2 Nearly half of the compounds on this list use quaternary ammonium as the active ingredient and a further 10% use quaternary ammonium in combination with other active ingredients. These formulations have been extensively evaluated; for a recent example, see Bondurant et al. (2019)1 and for activity against SARS-CoV-2, see Herdt et al. (2021).12
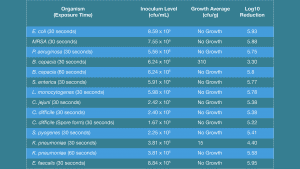
Given that the majority of hand sanitisers do not contain alcohol as the active ingredient, what are the other options apart from quaternary ammonium compounds? DGH Pharma has developed an innovative and novel preparation which relies on mechanical rather than biocide killing of bacterial cells and viruses. The active ingredients are metal ions, specifically copper and magnesium, in a nano-matrix formulation. Using this proprietary formulation against a range of bacterial species indicates an excellent bactericidal effect, with the majority of bacterial species completely inactivated in under 30 seconds of exposure, including spores of Clostridium difficile. A couple of bacterial species showed resistance to killing at 30 seconds of exposure, but these were completely inactivated by 60 seconds of exposure to the preparation. Results from these tests are shown in the table.
References
- Kampf, G et al. ‘Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents.’ The Journal of hospital infection vol. 104,3 (2020): 246-251. doi:10.1016/j.jhin.2020.01.022
- US Environmental Protection Agency. US EPA; Washington, DC: 2020. List N: disinfectants for use against SARS-CoV-2. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2
- Jain VM, Karibasappa GN, Dodamani AS, Prashanth VK, Mali GV. Comparative assessment of antimicrobial efficacy of different hand sanitisers: An in vitro study. Dent Res J (Isfahan). 2016;13(5):424-431. doi:10.4103/1735-3327.192283
- Siddharta, Anindya et al. ‘Virucidal Activity of World Health Organization-Recommended Formulations Against Enveloped Viruses, Including Zika, Ebola, and Emerging Coronaviruses.’ The Journal of infectious diseases vol. 215,6 (2017): 902-906. doi:10.1093/infdis/jix046
- Morton HE. The relationship of concentration and germicidal efficiency of ethyl alcohol. Ann N Y Acad Sci. 1950;53:191–196.
- Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents [published correction appears in J Hosp Infect. 2020 Jun 17;:]. J Hosp Infect. 2020;104(3):246-251. doi:10.1016/j.jhin.2020.01.022
- Kampf G, Kramer A, Suchomel M. Lack of sustained efficacy for alcohol-based surgical hand rubs containing ‘residual active ingredients’ according to EN 12791. J Hosp Infect. 2017 Feb;95(2):163-168. doi: 10.1016/j.jhin.2016.11.001. Epub 2016 Nov 12. PMID: 27912980.
- Blaney DD, Daly ER, Kirkland KB, Tongren JE, Kelso PT, Talbot EA. Use of alcohol-based hand sanitisers as a risk factor for norovirus outbreaks in long-term care facilities in northern New England: December 2006 to March 2007. Am J Infect Control. 2011;39:296–301.
- Vogel L. Hand sanitisers may increase norovirus risk. CMAJ 2011;183:E799.
- Rosenthal M, Goldberg D, Aiello A, Larson E, Foxman B. Skin microbiota: microbial community structure and its potential association with health and disease. Infect Genet Evol. 2011;11(5):839-848. doi:10.1016/j.meegid.2011.03.022
- Bondurant SW, Duley CM, Harbell JW. Demonstrating the persistent antibacterial efficacy of a hand sanitiser containing benzalkonium chloride on human skin at 1, 2, and 4 hours after application. Am J Infect Control. 2019 Aug;47(8):928-932. doi: 10.1016/j.ajic.2019.01.004. Epub 2019 Feb 16. PMID: 30777389