The Health Technology Program at Aarhus University applies computational biology to investigate the heterogeneity of tumours
Increasing computational power combined with enhanced access to imaging technologies brings mathematical analysis of biological events to the forefront of medicine. These combined technologies provide novel approaches to document the performance of medical devices and understand disease progression in virtual patients. As these methods mature we see regulatory authorities looking for computational medicine modelling technologies that support their decision making.1
The aim is to develop representative computer simulations in conjunction with appropriate validation methodologies that assure the credibility of the computational results and help devices enter the marketplace, utilising the least burdensome approach.
In disease progression, we have previously used these methods to study the risk of realising stroke2 in humans and exemplified how these computational medicine techniques can be used to gain insight into the progression of cancer emerging on the foot of a mouse.3
What challenges the mathematical modelling of biology is its great complexity. This is seen in the sophisticated structural layout of tissues and the dynamics of cells, all being regulated by the presence of a high number of reactive and different biochemical species. Biological events occurring in this system comply with the laws of physics described by differential equations.
Even though the governing equations in principle are known, the total number of equations needed for a complete description of an organism is often not possible to solve simultaneously. Therefore, a computational medicine model includes inherent approximations made to reach a result at all. Thus, models must be validated if we should maintain trust in them.
Imaging provides information that is fed into the computational models. By selecting the right imaging technology, you gain information on, for instance, the structural layout of the tissue, or the concentration levels of a specific biochemical molecule.
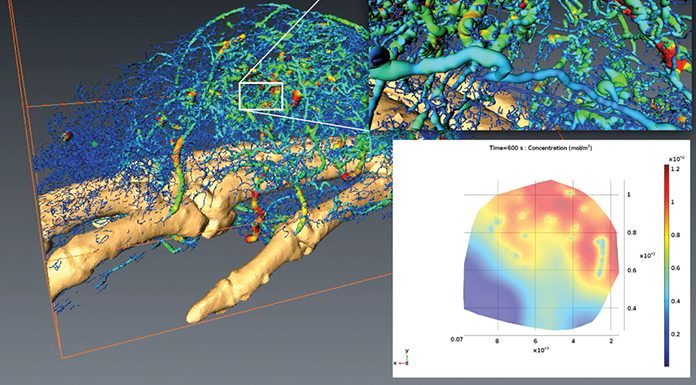
It is important to consider this at the appropriate length and timescale. We considered the 600 seconds after a drug is injected into a mouse.3 Using magnetic resonance imaging (MRI) the overall presence of the drug can be determined, but this technique does not allow for detailed mapping of the individual blood vessels.
Using micro-computed tomography (MCT), no information is obtained on the drug distribution, but a very precise description of the tissue is realised (see upper right insert in Fig. 1). Validation ensured that the overall presence of drug in the tumour is matched by the computational predictions.
Because the simulation includes all the complex structural information (as shown in the left of Fig. 1), it is now possible to precisely describe the local drug accumulation down to the length scale of a single cell (see lower right insert in Fig. 1).
We made a mathematical model describing molecular distribution based on detailed vascular network structures. The improved insight gained from the modelling of heterogeneous tumours helps us to further understand why every cancer cell may be unique and why personalised treatments are necessary.4
References
- Regulatory Science Priorities 2016, Center for Devices and Radiological Health, US Food and Drug Administration
2. Thrysoe et al. (2010) Stroke 41(5) 1041-1043
3. Wittenborn et al. (2016) Microvascular Research 108:69-74
4. Marate (2013) Nature Insight Tumour Heterogeneity 501:327-372