The incidence of cancer has dramatically increased over the last 50 years as a result of prolonged life expectancy and the Western lifestyle. Recent advances in current cancer care have reduced the mortality of many cancers and transformed some lethal ones to a manageable chronic disease.
Despite these achievements the majority of patients still succumb to their disease. For this reason, researchers and clinicians are continually striving to find new ways to develop the diagnosis and treatment of cancer for improved cancer prevention and cure.
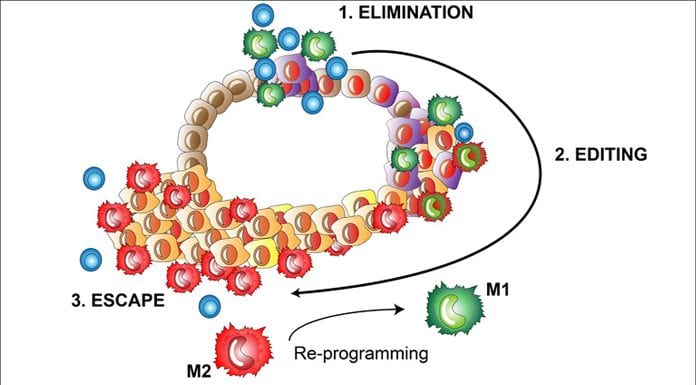
Cancer diagnosis can be considered as the tip of an iceberg and the underlying events that lead to the development of cancer are complex and involve a multistep process of malignant progression of cancer cells and immune recognition, as well as elimination of the transformed cells by our own immune system1 (Fig. 1). Cancers that have managed to grow under immune surveillance have undergone a process called immuno-editing where variants of the tumour cells gain properties to survive immune attack. During this phase, a multitude of tumour-intrinsic factors enable the cells to avoid immune recognition and several tumour-extrinsic mechanisms promote the formation of an immunosuppressive tumour micro-environment that leads to improper activation of tumour killing T cells and ultimately outgrowth of the tumour.
Immune cells versus cancer
The newest and most potent cancer treatments utilise the body’s own immune system to kill cancer cells. The idea is to re-activate anti-tumour immune responses by modulating the activity of signals enabling immune-escape. The current immunotherapy approaches mainly target the re-activation of tumour-specific T cells and include unselective immune activators (such as cytokines), cancer vaccines, adoptive T cell therapies, and immune checkpoint inhibitors. The immune checkpoint inhibitors targeting CTLA-4, PD-1, and PD-L1 have dramatically improved patient outcome in various cancer types and revolutionised our understanding of the significance of the immune system in cancer management.2
While T cell targeted immunotherapy works like magic for some patients, others show modest or no response at all. It is still largely unclear why this is; however, many cell types in the tumour micro-environment can prevent the anti-tumour functions of re-activated T cells. Macrophages are one of the most abundant cells in the tumour micro-environment that can be educated by cancer cells as a weapon against our own body. The reason for this is that most of the macrophages in tumours represent a phenotype that is normally found in wounds; they dampen inflammatory responses, induce tissue remodelling and promote the formation of new blood vessels.3 This is due to the influence of cancer secreted molecules and other stromal components on macrophage differentiation. All of the above-mentioned functions help tumours to grow and, importantly, can prevent current treatments from working properly.
Macrophages are very plastic cells and can be re-educated in the tumour micro-environment to support immune activation, which makes them an ideal target for improved cancer immunotherapy (Fig. 1). Macrophage-targeted therapies are still under clinical development but commercial interest towards this strategy has set deals of hundreds of millions (dollars) between pharma companies.
Most of the current strategies focus on inhibiting the macrophage growth factor receptor (CSF-1R), which, depending on the situation, leads either to complete macrophage depletion or to re-education of them to a pro-inflammatory phenotype. Already at this stage, several researchers, including us,4 have shown that, depending on the tumour micro-environment, macrophages are more likely to be promoted into pro-tumoral cells if their growth factor receptor signalling is blocked. This is because many other tumour micro-environmental molecules or other growth factor receptor agonists can replace signalling of the CSF-1 receptor when it is inhibited. When this occurs, the replacement signalling pathways may be even more harmful than CSF-1R signalling and promote differentiation of macrophages into tumour supporting cells. For this reason, new macrophage-phenotype modulating molecules are urgently needed as part of future immunotherapy.
Our research exploits a unique scavenger receptor Clever-1, expressed on a subpopulation of immunosuppressive macrophages, to re-activate anti-tumour immunity and develop Clever-1 as a companion therapeutic, diagnostic, and prognostic biomarker to treat and identify patients under immunosuppression. This involves the use of in vivo tumour models and sophisticated immunological assays with cutting-edge technology and state-of-the-art imaging combined with fresh human cancer patient material to elucidate the function of Clever-1 in controlling macrophage mediated local and systemic immune responses. Our results potentially have a high impact in understanding the mechanism of macrophage-mediated immunosuppression in cancer and promoting anti-Clever-1 immunotherapy into clinical trials where it may have benefits in combination with currently available immune activating drugs.
 Maija Hollmén is an adjunct professor of tumour immunology, group leader and academy research fellow at the MediCity Research Laboratory, Institute of Biomedicine, University of Turku, Finland.
Maija Hollmén is an adjunct professor of tumour immunology, group leader and academy research fellow at the MediCity Research Laboratory, Institute of Biomedicine, University of Turku, Finland.
Twitter: Hollmenlab: @maijahollmen; Maija Hollmen: @HollmenMaija
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74
- Xu-Monette ZY, Zhang M, Li J, Young KH. PD-1/PD-L1 Blockade: Have We Found the Key to Unleash the Antitumor Immune Response? Front Immunol 2017;8:1597
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014;41:49-61
- Hollmén M, Karaman S, Schwager S, Lisibach A, Christiansen AJ, Maksimow M, et al. G-CSF regulates macrophage phenotype and associates with poor overall survival in human triple-negative breast cancer. Oncoimmunology 2016;5:e1115177