Researchers at the University Hospital Basel discuss developments in the diagnosis and treatment of asthma and allergies
Asthma is defined as a chronic inflammatory disease of the lung with unknown origin. According to an increasing number of epidemiologic studies, the incidence of asthma cases is increasing worldwide. The EU Symposium on the Awareness of Allergy (2016) declared that ‘Chronic airway diseases are a major and growing health problem in Europe’ (Muraro 2017).
The World Health Organization (WHO) and the Health Effects Institute (State of Global Air 2017 Report, Boston) announced that the worldwide increase of asthma is linked to air pollution. However, the mechanism of how air pollution, or what type of air pollution, leads to asthma is yet to be investigated.
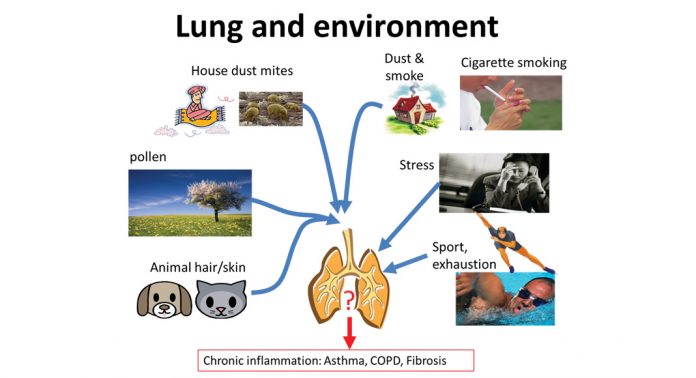
The patients experience asthma as chest tightness, shortness of breath and coughing caused by certain inhaled substances, physiological, physical, or psychological stress. Allergic asthma is diagnosed when the patient responds to a known allergen, which does not necessarily have to be inhaled, but can also be taken up with food or by skin contact. Non-allergic asthma can be caused by sudden changes of air humidity, temperature, air pressure or by exercise or excitement/stress. Neither the mechanism by which this wide range of triggers can initiate asthma, nor how they provoke an asthma attack, is well understood (Fig. 1).
In most medical text books, and in the new research guidelines of the European Respiratory Society on asthma pathogenesis, the allergic aspect and inflammation are the focus, ignoring the fact that tissue remodelling occurs, manifested as hyperplasia and hypertrophy in the large or medium-sized airways in most asthma patients. The most prescribed drugs for asthma therapy are inhaled steroids and long-acting β2-agonists. Steroids are the most effective anti-inflammatory compounds which rapidly reduce airway inflammation.
Long-acting β2-agonists relax the constricted airway muscles after an asthma attack and thereby ease breathing. In clinical trials it was convincingly demonstrated that the combination of both classes of drugs is more potent than an increased dosage of either drug alone. The molecular mechanism of this beneficial combination on inflammation in airway smooth muscle cells was investigated and described by us between 1999 and 2009.
During the past decade, humanised antibodies have been developed and applied to treat allergic diseases including asthma. Each antibody targets a specific inflammatory mediator and neutralises its action. However, clinical studies on anti-IL-5 and anti-IgE antibodies showed that the therapeutic effects of these new drugs is limited to a relatively small number of asthma patients and cannot substitute inhaled steroids.
Furthermore, the introduction of these drugs did not reduce the costs to the healthcare system for asthma patients, and they remain too expensive for most patients in less-developed countries. Similar to inhaled steroids and long-acting β2-agonists, none of these drugs have any proven long-term effect on airway wall remodelling.
Today, bronchial thermoplasty is the only therapy, which lastingly reduces airway wall remodelling by decreasing the mass of airway smooth muscle cells. This treatment is only used for patients with severe or difficult to control asthma and only a limited number of clinics have access to the equipment and the technique. The majority of patients treated with bronchial thermoplasty reduce the use of inhaled steroids, and have fewer hospitalisations and longer symptom-free periods over several years.
What is known on airway wall remodelling in asthma
Contrary to the long-held hypothesis, airway wall remodelling is not the result of chronic inflammation, but can be induced within four days (Grainge 2011, Ijpma 2017, Rao 2017). The major pathological change is the increase of the epithelial cell layers and of smooth muscle mass, which is re-arranged, forming a spiral-like structure around the bronchi, as was first documented in the rhesus monkey model for asthma (Tran 2004).
This observation may explain the failure of other studies to link smooth muscle increase in asthma to hyper-responsiveness of the airways during an attack. A spiral of muscle will constrict more forcefully than muscles arranged in a different way.
Therefore, the measurement of smooth muscle mass alone is insufficient to explain hyper- responsiveness of the asthmatic airway, the muscle arrangement around the airway should also be determined.
Childhood asthma
Combining the above results with new studies in childhood asthma clearly indicated that airway wall remodelling is not the result of chronic inflammation, but is an independent pathology that causes dysfunction of the airways in asthma. In addition, the activated smooth muscle cells release pro-inflammatory cytokines, which attract mast cells, macrophages, eosinophils and neutrophils into the airways, thereby exacerbating inflammation.
There are indications that airway wall remodelling results from a loss of communication between tissue cells which form the airway, including epithelial cells, fibroblasts and smooth muscle cells.
The communication between these cells was assigned as the epithelial-mesenchymal-trophic-unit (EMTU), first described by Davies and Holgate (2002). Specific growth factors and cytokines have been studied in this context of cell-cell communication, but we are far from understanding how the large variety of proteins controlling the EMTU affect each other, or how the lack of one leads to asthma. The complexity of the EMTU is reflected in the fact that airway wall remodelling consists of several events, which can occur independent of each other, including:
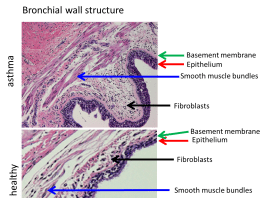
- the loss of the integrity of the epithelium which lines the bronchi;
- thickening of the basement membrane which separates the epithelium from fibroblasts and smooth muscles;
- increased deposition of extracellular matrix in the sub-epithelial cell layers;
- modified composition of the extracellular matrix forming a pro-inflammatory condition for fibroblasts and smooth muscles;
- increased vascularisation of the sub-epithelial cell layers;
- sub-epithelial fibrosis; and
- increased smooth muscle mass caused by hypertrophy and hyperplasia (Fig. 2).
The available literature neither allows any conclusion to be drawn on which of these events occur first, nor which of these events may lead to another. Unfortunately, none of the animal models (except rhesus macaque) can reproduce asthma as it occurs in humans, since only limited pathologies of asthma can be induced experimentally on any of the animal models. Therefore, new therapies that work on an animal model often failed when applied to therapy in humans.
Our research
Using isolated primary human airway cells obtained from patients with asthma, COPD or fibrosis, we were able to provide initial explanations on the molecular biological level for the pathogenesis of each disease, as well as for the beneficial actions of new drugs. In regard to asthma, we were the first to explain the molecular mechanism that underlays the clinically well-documented beneficial combination of inhaled steroids with long-acting β2-agnonists.
The combined drugs synchronised transcription factors resulting in an improved efficacy of anti-inflammatory and anti-proliferative action. In asthma, however, the anti-proliferative action of the drugs is significantly reduced due to the lack of transcription factors which regulate smooth muscle differentiation and function. As a result, the airway smooth muscle cells of patients proliferate faster than those of healthy individuals, leading to remodelling.
Furthermore, we provided the first evidence that the lack of at least two relevant well-known pro-inflammatory and remodelling regulating proteins is due to faulty translation. Therefore, the genetic information is not adequately translated into proteins. These cell type specific mediators of inflammation and remodelling can be downregulated by well-known triggers, such as house dust mites, cigarette smoke and IgE, providing the mechanism behind airway wall remodelling in asthma.
In collaboration with our Chinese colleagues (Xi’an Jiaotong University, PR China), we reported the constitutive up-regulation of a novel enzyme, PRMT1, which is regulated by C/EBPs on the level of epi-genetic control (Sun 2017). This constitutive
over-expression of PRMT1 in airway smooth muscle cells and fibroblasts is disease-specific and leads to increased proliferation, extracellular matrix deposition and mitochondrial activity. These findings may explain the regulatory mechanism leading to these asthma-associated pathologies.
In theory, the lack of C/EBP-α as well as the over-expression of PRMT1 could be targeted by existing small peptide drugs. However, the challenge is the delivery of such drugs in a cell type specific manner. In order to avoid unwanted side effects, further studies have to be carried out to investigate the effect of such peptide drugs on other cells of the airway wall which already have sufficient C/EBP-α or have no over-expression expression of PRMT1.
Together with our colleagues from Sydney University (Australia), we documented that the composition of the extracellular matrix in the airway wall of patients is modified in a way that it promotes inflammation, compared to healthy individuals.
Together with our colleagues at Manitoba (Canada), Sydney (Australia) and Thessaloniki (Greece), we provided data from our human cell model that steroids and β2-agonists modify signalling and the composition of extracellular matrix in a drug and disease specific manner. Unfortunately, these studies showed that inhaled steroids may further promote remodelling.
However, all drugs improved the expression of beneficial, anti-inflammatory components of the extracellular matrix (high molecular weight hyaluronic acid) in both asthma and COPD patients. The missing part of these studies is the analysis of the net result in the diseased airway in patients.
Such an investigation is very difficult since ethical approval cannot be justified in humans.
Key message
The incidence of asthma is increasing worldwide without any known reason. it is not curable, and only the control of symptoms can be achieved with available therapies, which are mainly targeting inflammation and airway constriction. According to the European Symposium on the Awareness of Allergy (2016), over 50% of patients are often wrongly diagnosed, ignored or incorrectly treated in Europe.
Airway wall remodelling is a major and most probably the causative pathology of the affliction and neither the cause nor the consequences of airway wall remodelling are well understood. In 2015, Saglani and Lloyd stated in the European Respiratory Journal: ‘…unless the mechanism driving airway wall remodelling is better understood, there will be no cure for asthma’.
It has to be emphasised that none of the available drugs have any significant effect on airway wall remodelling.
Our lack of knowledge on airway wall remodelling in asthma is reflected in a literature search (Pubmed, May 2017) which resulted in a total of 164,531 publications related to asthma. Of these, 32,427 focused on inflammation and immune response, while only 3,858 investigated tissue remodelling (2,561 in the last ten years).
In a more detailed analysis by the American Thoracic Society, it was concluded that the low number of available data is often used by funding organisations to reject projects on airway wall remodelling with the argument that there is little evidence for its impact on asthma (Prakash 2017).
We and our international collaborators have contributed substantial new knowledge over the last 20 years to understand the impact of airway wall remodelling in the pathology of asthma. Unfortunately, the development of novel therapeutic strategies to this end has been hampered by a lack of relevant research calls.
Hopefully, politicians and strategy planners in the industry will recognise this gap and provide urgently needed financial resources to help millions of people worldwide.
References
Davies DE, Holgate ST. Asthma: the importance of epithelial mesenchymal communication in pathogenesis. Inflammation and the airway epithelium in asthma. Int J Biochem Cell Biol. 2002;34:1520-6
Grainge CL, et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011;364:2006-15
Ijpma G, et al. Directional preference of airway smooth muscle mass increase in human asthmatic airways. Am J Physiol Lung Cell Mol Physiol. 2017 doi:10.1152/ajplung.00353.2016
Rao SS, et al. Calpain-activated mTORC2/Akt pathway mediates airway smooth muscle remodelling in asthma. Clin Exp Allergy. 2017;47:176-189
Saglani S, Lloyd CM. Novel concepts in airway inflammation and remodelling in asthma. Eur Respir J. 2015;46:1796-804
Sun Q, et al. Constitutive high expression of protein arginine methyltransferase 1 in asthmatic airway smooth muscle cells is caused by reduced microRNA-19a expression and leads to enhanced remodeling. J Allergy Clin Immunol. 2017 doi:10.1016/j.jaci.2016.11.013
Tran MU, et al. Smooth muscle hypertrophy in distal airways of sensitized infant rhesus monkeys exposed to house dust mite allergen. Clin Exp Allergy. 2004;34:1627-33